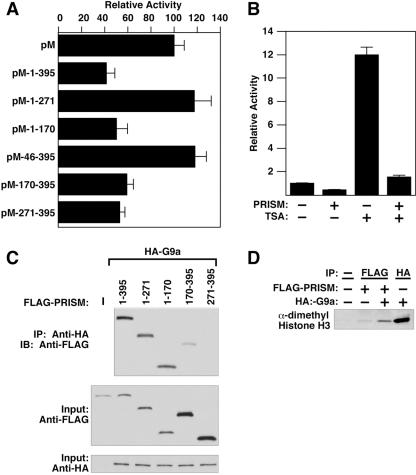
FIG. 5.
PRISM contains two repressive domains and recruits methyltransferase activity. (A) The PR and Zn finger domains of PRISM repress transcription. Cotransfections of the 5× GAL4-UAS luciferase reporter and either the GAL4 DBD alone (pM) or GAL4-PRISM (100 ng) constructs are shown. All data are normalized to protein content as described above. (B) Histone deacetylase inhibitors do not abrogate PRISM-mediated repression. COS-7 cells were cotransfected with 5× GAL4-UAS and either the GAL4 DBD alone or GAL4-PRISM (100 ng) and treated with 100 nM TSA for 24 h prior to harvest. (C) Immunoprecipitations (IP) of PRISM with G9a. Cotransfected lysates were incubated with anti-HA (G9a) antibody followed by immunoblotting (IB) with anti-FLAG (PRISM). (D) PRISM associates with HMT activity. Results from histone methylation assays from transfected cell lysates are shown. Cells were transfected with PRISM, G9a, or both and subjected to immunoprecipitation using the designated antibody and subsequent methyltransferase assays. All samples containing PRISM were immunoprecipitated with anti-FLAG (PRISM) antibodies. The amino acid positions of the deletion constructs are indicated.