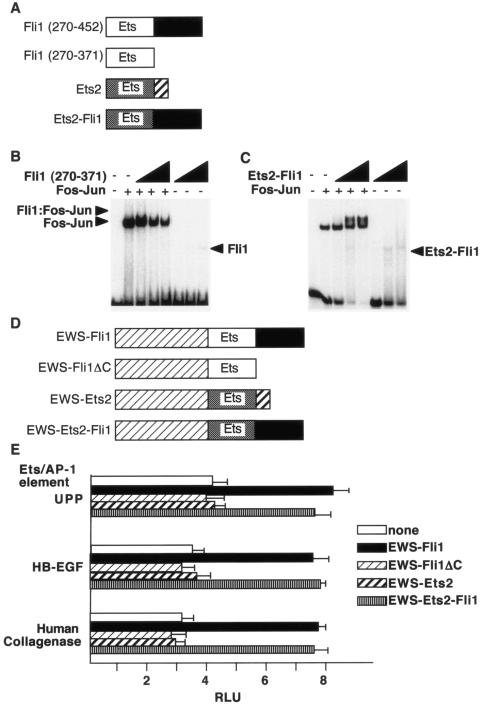
FIG. 7.
The C terminus of Fli1 is required for cooperative DNA binding. (A) Schematic diagrams of the indicated Ets proteins. (B) The isolated Fli1 Ets domain does not cooperatively bind DNA. Shown is a gel shift assay with Fli1 (amino acids 270 to 371) and Fos-Jun on the tandem elements of the human collagenase promoter. The concentrations of Fli1 were 35.3, 141, and 565 nM. (C) An Ets2-Fli1 fusion protein binds DNA cooperatively with Fos-Jun. A fusion protein containing the Ets domain of Ets2 and the C-terminal sequences of Fli1 was used in gel shift assays with the tandem elements of the human collagenase promoter as a probe. Protein concentrations were as described above. (D) Schematic diagrams of the indicated EWS-Ets fusion proteins. (E) The indicated EWS-Ets fusion proteins were cotransfected with reporter genes harboring the indicated tandem elements into 3T3 cells. At 40 h after transfection, luciferase activity was determined. RLU, relative light units.