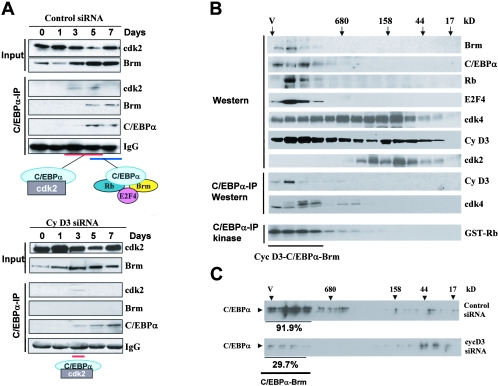
FIG. 6.
Phosphorylation of C/EBPα by cyclin D3-cdk4/cdk6 is required for the formation of the C/EBPα-cdk2 and the C/EBPα-Brm complexes. A. Examination of C/EBPα-cdk2 and C/EBPα-Brm complexes during differentiation of 3T3-L1 cells treated with control siRNA (upper) and with siRNA to cyclin D3 (bottom). Input lanes: Western blotting of nuclear extracts with antibodies to Brm and cdk2, showing the levels of these proteins in extracts used for the co-IP. C/EBPα-IP lanes: C/EBPα was immunoprecipitated from nuclear extracts, and the IPs were examined by Western blotting with antibodies to cdk2, Brm, and C/EBPα. Heavy chains of immunoglobulin G (IgG) are shown for each IP. B. Examination of the cyclin D3-C/EBPα-Brm complex in differentiated 3T3-L1 cells by size exclusion chromatography. Nuclear extracts from differentiated 3T3-L1 cells (day 7) were fractionated with an SEC-400 column. Fractions were analyzed by Western blotting with antibodies to the proteins shown on the right. C/EBPα-IP Western lanes: examination of cyclin D3 and cdk4 in C/EBPα IPs from size exclusion fractions was performed with specific antibodies as described in the legends to Fig. 3 and 4. Kinase activity of cyclin D3-cdk4 was examined in C/EBPα IPs by an in vitro kinase assay with Rb substrate (C/EBPα-IP kinase). C. Size exclusion chromatography of nuclear extracts isolated from 3T3-L1 cells treated with control siRNA and with cyclin D3 siRNA. Proteins were isolated at day 7 after initiation of differentiation and fractionated on an SEC-400 column. Size exclusion fractions were examined by Western blotting with antibodies to C/EBPα. Amounts of C/EBPα were calculated in fractions containing C/EBPα-Brm complexes relative to total amounts of C/EBPα throughout the gel filtration.