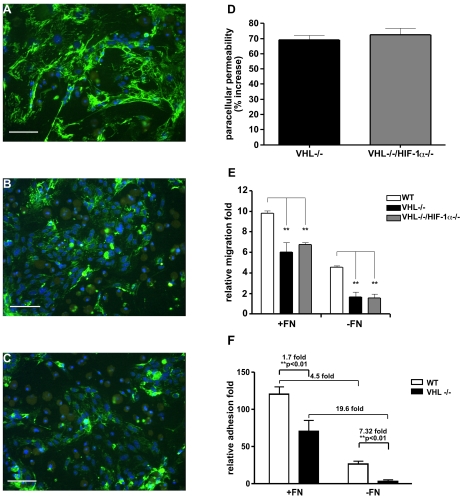
FIG. 6.
Defective extracellular fibronectin matrix assembly by VHL−/− and VHL-null HIF-1α-null ECs. (A to C) Wild-type (A), VHL−/− (B), and VHL−/− HIF-1α−/− (C) ECs were grown on coverslides in fibronectin-depleted medium for 1 week. Fibronectin (green color) was detected by indirect immunofluorescence staining using polyclonal antifibronectin antisera. Cell numbers were visualized with DAPI staining (blue color). Both VHL−/− (B) and VHL−/− HIF-1α−/− (C) ECs assemble very few fibronectin fibrils compared to wild-type ECs (A). Bar, 100 μm. (D) Permeability across the EC monolayer. VHL−/− (black bar) and VHL−/− HIF-1α−/− (gray bar) confluent ECs show a >70% increased passage of FITC-dextran compared with wild-type ECs (mean ± SEM). (E) Migration of ECs was analyzed in a Boyden chamber. Wild-type (white bars), VHL−/− (black bars), and VHL−/− HIF-1α−/− (gray bars) ECs were plated in transwells either coated with fibronectin (+FN) or coated with collagen (−FN). The migrating cells were stained as described in Materials and Methods (results are shown as the mean ± SEM). (F) Adhesion assay. Forty-eight-well plates were coated with either 5-μg/well fibronectin (+FN) or PBS (−FN). A total of 5 × 104 EC in 200 μl serum-free adhesion medium were plated in the wells and allowed to attach for 1 h. Wild-type ECs (white bars) and VHL-null ECs (black bars) were then washed gently, and the adherent cells were stained with 0.1% crystal violet solution. The dye was dissolved in methanol, and the absorbance of the solution was read at 595 nm in an enzyme-linked immunosorbent assay plate reader (mean ± SEM). Statistical analysis was performed using the unpaired Student's test. *, P < 0.05; **, P < 0.01.