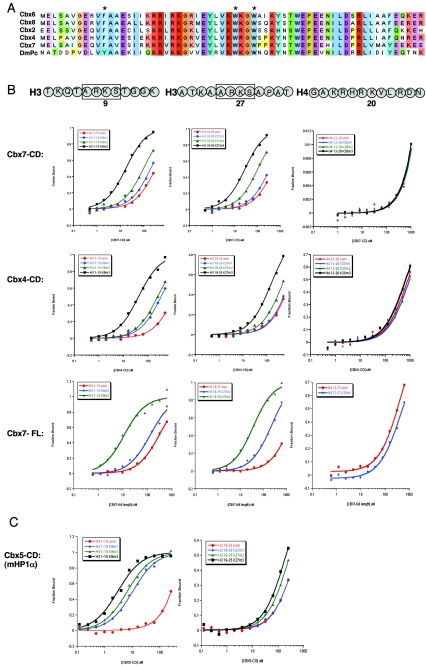
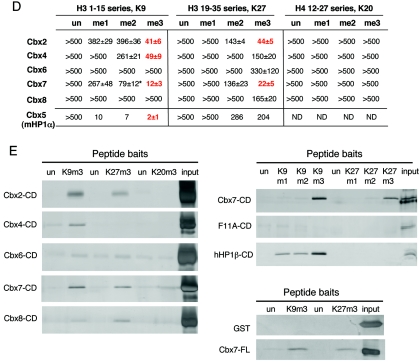
FIG. 1.
Analysis of Pc-like Cbx CD binding affinities for trimethylated H3K9 and H3K27. (A) ClustalW alignment of the five mouse Pc-like CDs (aa 1 to 62) and the Drosophila Pc CD (aa 16 to 78). The asterisks represent the caging aromatic residues that mediate the histone methyl-lysine interaction. Note the high degree of conservation among these family members. (B) Fluorescence polarization of Cbx7 (top) and Cbx4 (middle) to histone tail peptides, including the me1, me2, and me3 states on residues K9 and K27 of H3, and K20me1, K20me2, and K20me3 of H4, respectively. Full-length Cbx7 (bottom) was tested against all of the above except for the monomethylated forms of H3K9 and H3K27, H4K20me2 and H4K20me3, and behaves identically to its CD for peptides tested. Histone tail sequences are represented above; note the ARKS motifs of H3K9 and H3K27. See panel D for actual peptides used. (C) Fluorescence polarization of Cbx5 (mouse HP1α) to H3K9 and H3K27 histone tail peptides in the me1, me2, and me3 states. (D) Dissociation constants (Kd, in micromolars) for Cbx2, Cbx4, Cbx6, Cbx7, and Cbx8, as well as Cbx5 (mHP1α), with each series of methylated peptides for each backbone shown. Low-micromolar binding constants are highlighted in red. The asterisk depicts weak binding of Cbx7 for H3K9me2 (a mark that is enriched on the Xi). Values represent averages ± standard deviations for at least three independent experiments in all cases (except for Cbx5 with certain peptides). ND, not determined. (E) Peptide pull-down assays. (Left) All CDs were examined for binding to unmodified and trimethylated peptides representing H3K9, H3K27, and H4K20. Results support those obtained by FP (D). (Right) Cbx7 CD, Cbx7 caging aromatic point mutant F11A, and human HP1β CD recombinant proteins were tested for the ability to bind unmodified and me1, me2, and me3 peptides of H3K9 and K27. Note the trimethyl specificity of Cbx7. (Right, bottom) GST and full-length Cbx7 were examined for binding to unmodified and trimethylated biotinylated peptides of H3K9 and H3K27. Full-length Cbx7 behaves the same as its CD alone. GST does not bind any peptide, as expected. un, unmodified.