Figure 2.
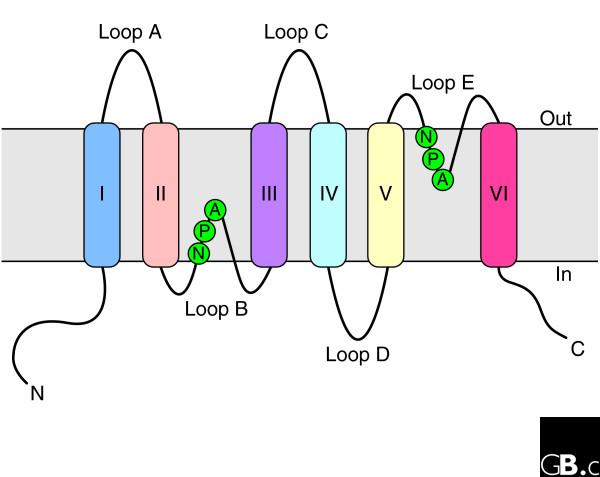
Topology of an aquaporin protein within the membrane. The protein consists of six transmembrane helices (I-VI) connected by five loops (A-E) and includes two internal tandem repeats (I-III and IV-VI, respectively). Loops B and E, containing the conserved NPA motifs (in the single-letter amino-acid code), form short α helices that fold back into the membrane from opposite sides. C, carboxyl terminus; N, amino terminus.
