Figure 5.
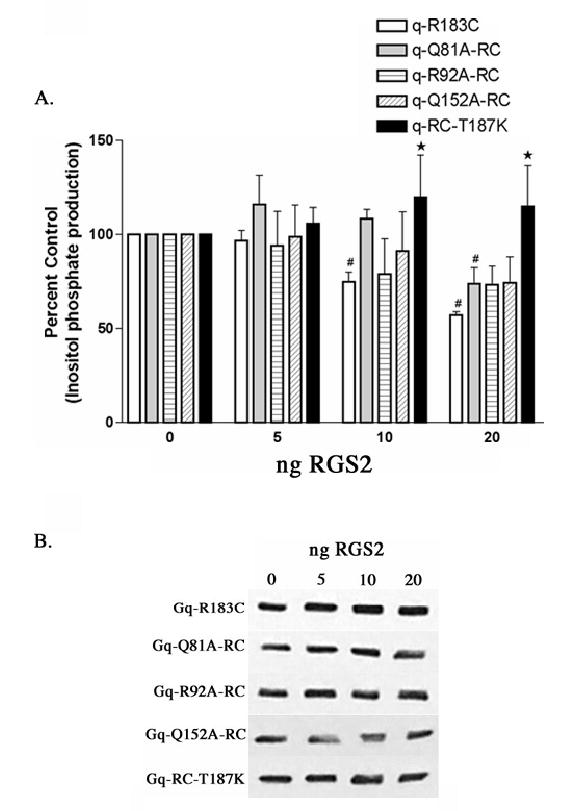
RGS2 inhibition of inositol phosphate production stimulated by Gαq-RC and Gαq-RC mutants. (A) HEK-293 cells were transfected with 0.1 μg of the constitutively active Gαq-R183C, Gαq-Q81A/RC, Gαq-R92A/RC, Gαq-Q152A/RC, or Gαq-RC/T187K and 0.2 μg of myc, His-tagged Gβ and 0.1 μg of Gγ and increasing amounts of RGS2 and empty vector up to a total of 1.0 μg of DNA. 24 hrs after transfection the cells were labeled with 2 μCi/ml myo-[3H]inositol and 16 hours later inositol phosphate production was determined, as described in Experimental Procedures. The results shown are averages from three independent experiments each done in triplicate and displayed as percent control + SD. The control is the inositol phosphate production stimulated by each mutant in the absence of any co-expressed RGS2. The statistical significance of the difference between the indicated bar and Gαq-R183C, in the absence of any additional mutations, transfected with equal amounts of RGS2 is denoted by ⋆ (p < 0.05) by oneway ANOVA followed by a Dunnett post-test. A (#) indicates a statistically significant difference (p < 0.05) by one-way ANOVA followed by a Dunnett post-test, between the indicated bar and the control, either Gαq-RC or a Gαq-RC mutant in the absence of cotransfected RGS2. (B) Western blots of total cellular lysates from a representative inositol phosphate experiment from (A) probed with the EE monoclonal antibody showing that increasing RGS2 expression does not effect expression of Gαq-RC or any of the mutants. We were not able to detect the level of RGS2 overexpression in these experiments; however the decrease in inositol phosphate production suggests that RGS2 expression was increased.
