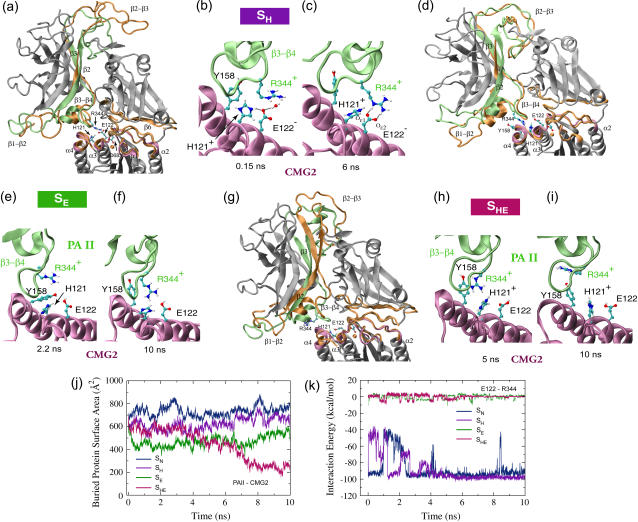
FIGURE 5.
Conformational changes of the anthrax-receptor complex under acidic conditions and interactions of key residues at the PAII-CMG2 binding surface. (a) Cartoon images of the initial and equilibrated SH structures in which all eight histidines are protonated. The color scheme and the alignment method are the same as in Fig. 2 a. (b–c) Despite initial disruption, the Arg-344PA-Glu-122CMG2 salt bridge is stably reformed after 5 ns equilibration. (d) Two overlaid cartoon snapshots of the SE structure where Glu-122CMG2 and all histidines, but His-121CMG2, are protonated. The snapshot taken at 2.2 ns is colored in the same scheme as the starting structure in a. The final structure reached during the equilibration is shown in orange. (e) The Arg-344PA-Glu-122CMG2 salt bridge is no longer formed. However, (f) Arg-344PA stays in the proximity of His-121CMG2. (g) Cartoon representations of the starting and 10-ns-equilibrated SHE structures with Glu-122CMG2 and all eight histidines protonated. In this case (h–i), Arg-344PA separates gradually from Glu-122CMG2. (j) Change of BPSA between PAII and CMG2 over the course of equilibrations of structures SN, SH, SE, or SHE. (k) Profiles of the Arg-344PA-Glu-122CMG2 interaction energy from these four equilibrations.