Abstract
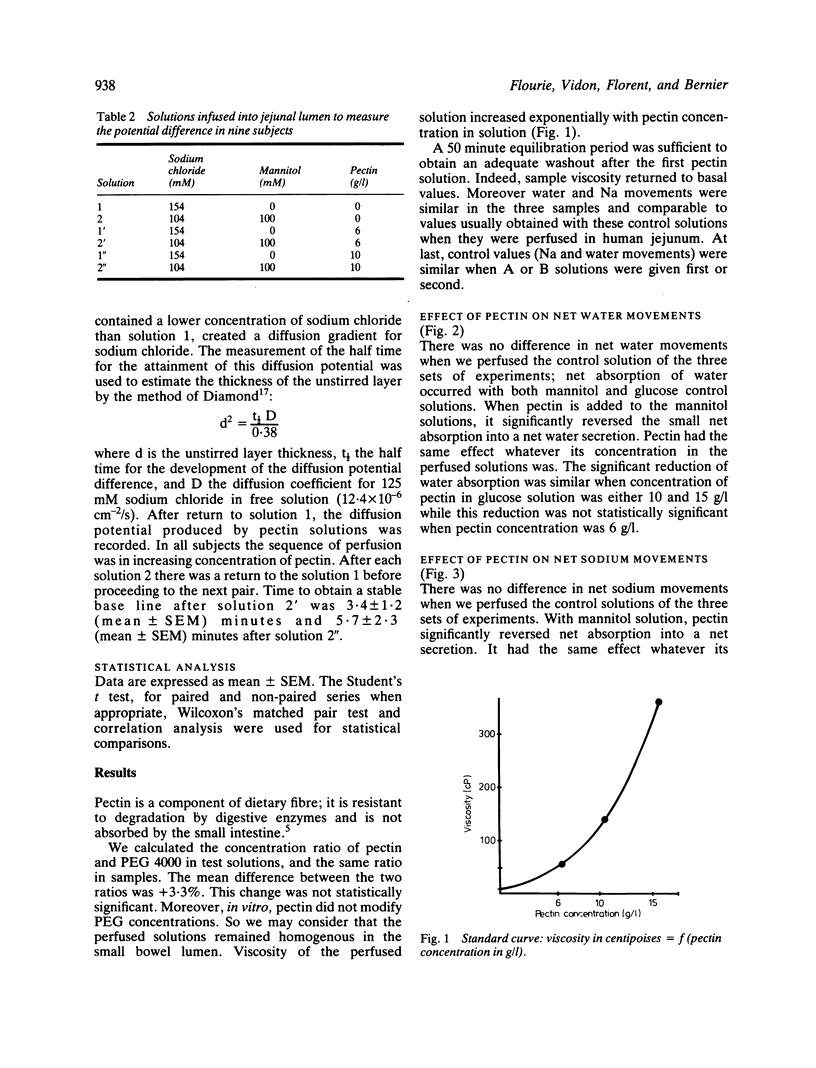
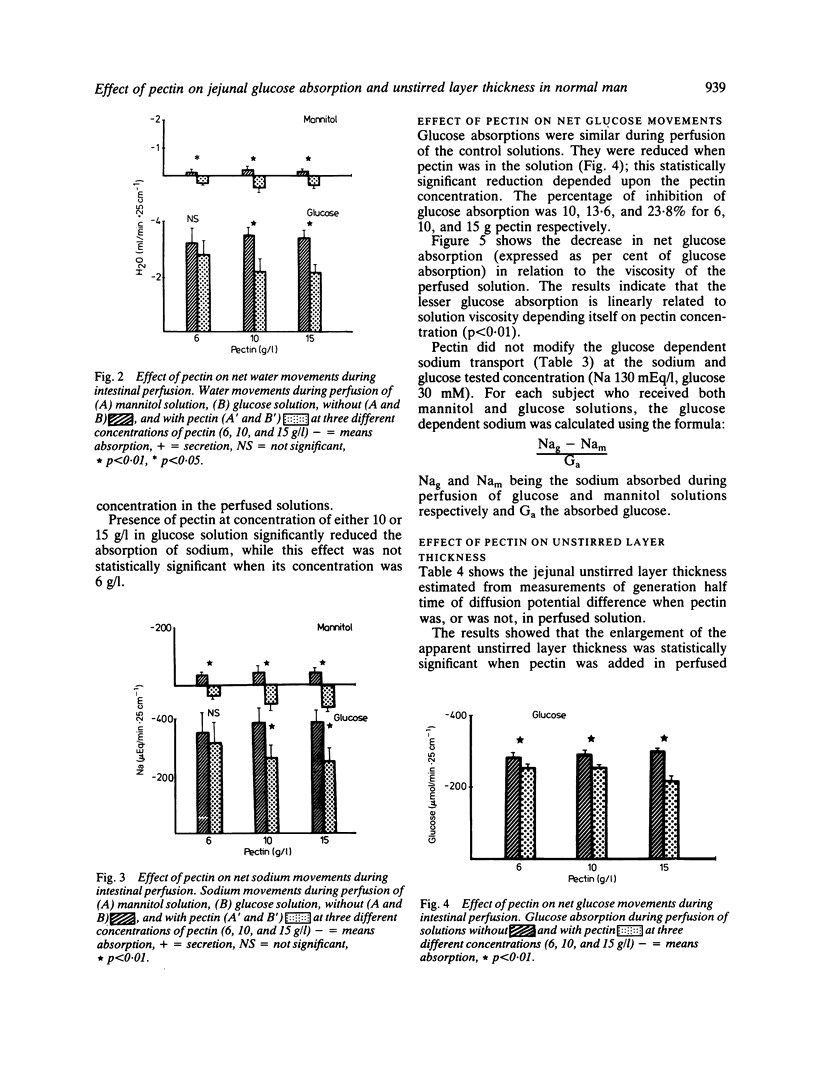
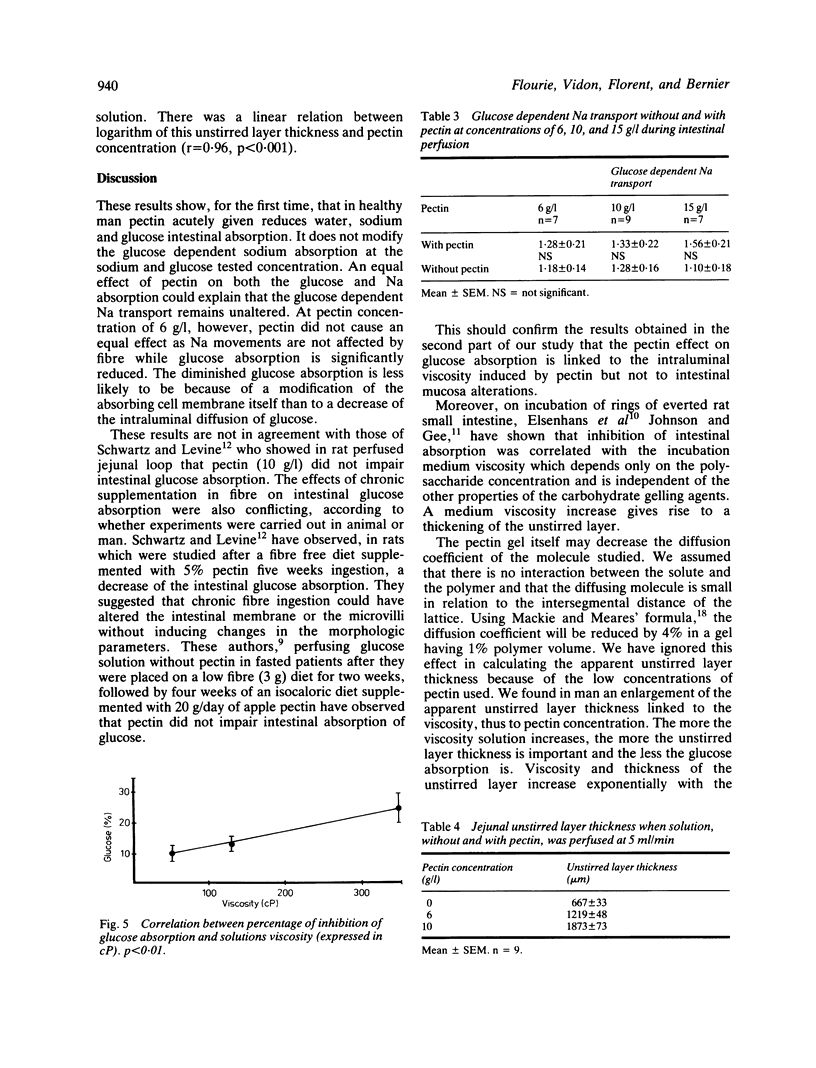
The effect of high methoxy apple pectin, a carbohydrate gelling agent, on the intestinal absorption of glucose, water, and sodium was studied in man. The effect of intraluminal fibre was evaluated in 22 healthy volunteers by the intestinal perfusion technique under an occlusive balloon. The test solutions (NaCl 130 mM, KCl 5 mM, glucose or mannitol 30 mM, PEG 4000 5 g/l) were perfused just beyond the ligament of Treitz at a rate of 10 ml/min. A 25 cm segment was studied. Three concentrations of pectin were tested: 6, 10, and 15 g/l. The effect of this pectin at two concentrations, 6 and 10 g/l, on the jejunal unstirred layer thickness was evaluated in nine other healthy subjects by an electrical technique. In mannitol solution, pectin reversed water and sodium absorption, whatever its concentration was, while in glucose solution it significantly reduced absorption of water and sodium at 10 and 15 g/l only (p less than 0.01). It significantly reduced glucose absorption at all concentrations (p less than 0.01). This reduction was found to be correlated with the solution viscosity (p less than 0.01). Pectin did not alter the glucose dependent sodium transport but increased significantly (p less than 0.001) the unstirred layer thickness. These results suggested that, in healthy man, pectin acutely given may impair intestinal absorption by means of an increased unstirred layer resistance. This effect could contribute to the diminished postprandial glycaemia observed in human subjects fed pectin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Midgley W. R., Wedman B. Fiber and diabetes. Diabetes Care. 1979 Jul-Aug;2(4):369–377. doi: 10.2337/diacare.2.4.369. [DOI] [PubMed] [Google Scholar]
- Diamond J. M. A rapid method for determining voltage-concentration relations across membranes. J Physiol. 1966 Mar;183(1):83–100. doi: 10.1113/jphysiol.1966.sp007852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenhans B., Süfke U., Blume R., Caspary W. F. The influence of carbohydrate gelling agents on rat intestinal transport of monosaccharides and neutral amino acids in vitro. Clin Sci (Lond) 1980 Nov;59(5):373–380. doi: 10.1042/cs0590373. [DOI] [PubMed] [Google Scholar]
- Holt S., Heading R. C., Carter D. C., Prescott L. F., Tothill P. Effect of gel fibre on gastric emptying and absorption of glucose and paracetamol. Lancet. 1979 Mar 24;1(8117):636–639. doi: 10.1016/s0140-6736(79)91079-1. [DOI] [PubMed] [Google Scholar]
- Jenkins D. J., Gassull M. A., Leeds A. R., Metz G., Dilawari J. B., Slavin B., Blendis L. M. Effect of dietary fiber on complications of gastric surgery: prevention of postprandial hypoglycemia by pectin. Gastroenterology. 1977 Aug;73(2):215–217. [PubMed] [Google Scholar]
- Jenkins D. J., Goff D. V., Leeds A. R., Alberti K. G., Wolever T. M., Gassull M. A., Hockaday T. D. Unabsorbable carbohydrates and diabetes: Decreased post-prandial hyperglycaemia. Lancet. 1976 Jul 24;2(7978):172–174. doi: 10.1016/s0140-6736(76)92346-1. [DOI] [PubMed] [Google Scholar]
- Jenkins D. J., Leeds A. R., Gassull M. A., Cochet B., Alberti G. M. Decrease in postprandial insulin and glucose concentrations by guar and pectin. Ann Intern Med. 1977 Jan;86(1):20–23. doi: 10.7326/0003-4819-86-1-20. [DOI] [PubMed] [Google Scholar]
- Jenkins D. J., Wolever T. M., Leeds A. R., Gassull M. A., Haisman P., Dilawari J., Goff D. V., Metz G. L., Alberti K. G. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. Br Med J. 1978 May 27;1(6124):1392–1394. doi: 10.1136/bmj.1.6124.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson I. T., Gee J. M. Effect of gel-forming gums on the intestinal unstirred layer and sugar transport in vitro. Gut. 1981 May;22(5):398–403. doi: 10.1136/gut.22.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds A. R., Gassull M. A., Metz G. L., Jenkins D. J. Letter: Food: influence of form on absorption. Lancet. 1975 Dec 13;2(7946):1213–1213. doi: 10.1016/s0140-6736(75)92706-3. [DOI] [PubMed] [Google Scholar]
- Miranda P. M., Horwitz D. L. High-fiber diets in the treatment of diabetes mellitus. Ann Intern Med. 1978 Apr;88(4):482–486. doi: 10.7326/0003-4819-88-4-482. [DOI] [PubMed] [Google Scholar]
- Modigliani R., Bernier J. J. Absorption of glucose, sodium, and water by the human jejunum studied by intestinal perfusion with a proximal occluding balloon and at variable flow rates. Gut. 1971 Mar;12(3):184–193. doi: 10.1136/gut.12.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read N. W., Barber D. C., Levin R. J., Holdsworth C. D. Unstirred layer and kinetics of electrogenic glucose absorption in the human jejunum in situ. Gut. 1977 Nov;18(11):865–876. doi: 10.1136/gut.18.11.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. E., Levine G. D. Effects of dietary fiber on intestinal glucose absorption and glucose tolerance in rats. Gastroenterology. 1980 Nov;79(5 Pt 1):833–836. [PubMed] [Google Scholar]
- Schwartz S. E., Levine R. A., Singh A., Scheidecker J. R., Track N. S. Sustained pectin ingestion delays gastric emptying. Gastroenterology. 1982 Oct;83(4):812–817. [PubMed] [Google Scholar]
- Trowell H. Crude fibre, dietary fibre and atherosclerosis. Atherosclerosis. 1972 Jul-Aug;16(1):138–140. doi: 10.1016/0021-9150(72)90017-2. [DOI] [PubMed] [Google Scholar]
- Whalen G. E., Harris J. A., Geenen J. E., Soergel K. H. Sodium and water absorption from the human small intestine. The accuracy of the perfusion method. Gastroenterology. 1966 Dec;51(6):975–984. [PubMed] [Google Scholar]


