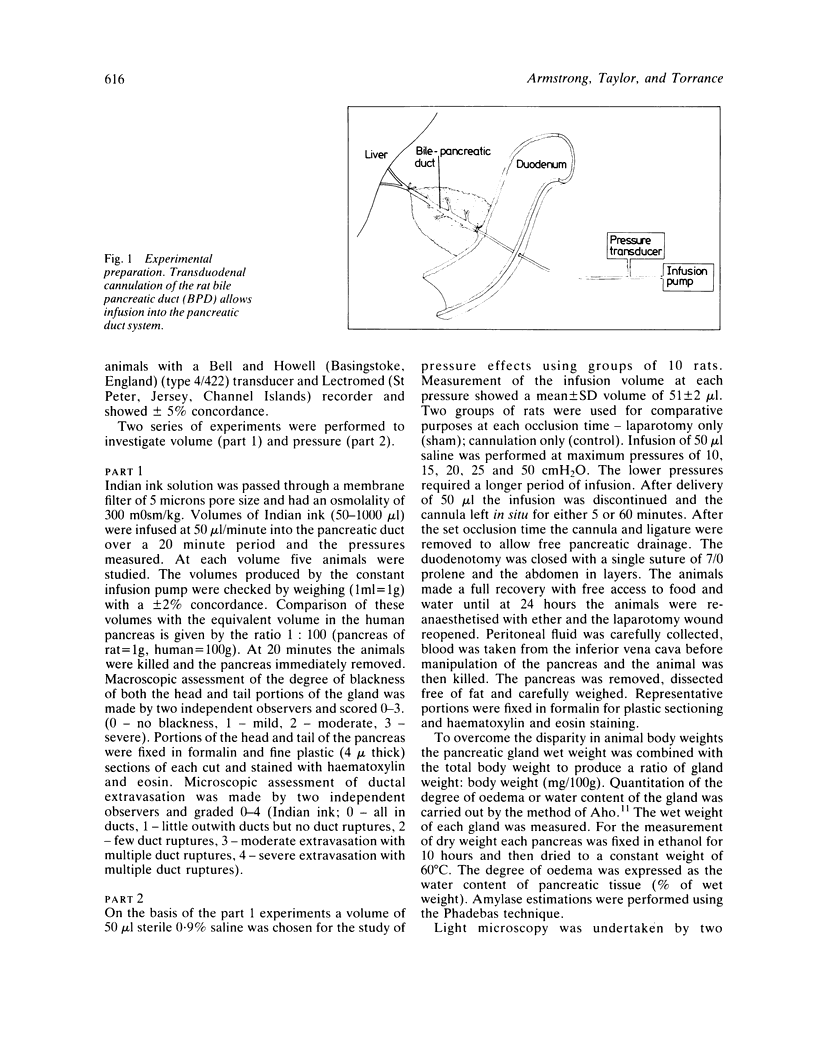
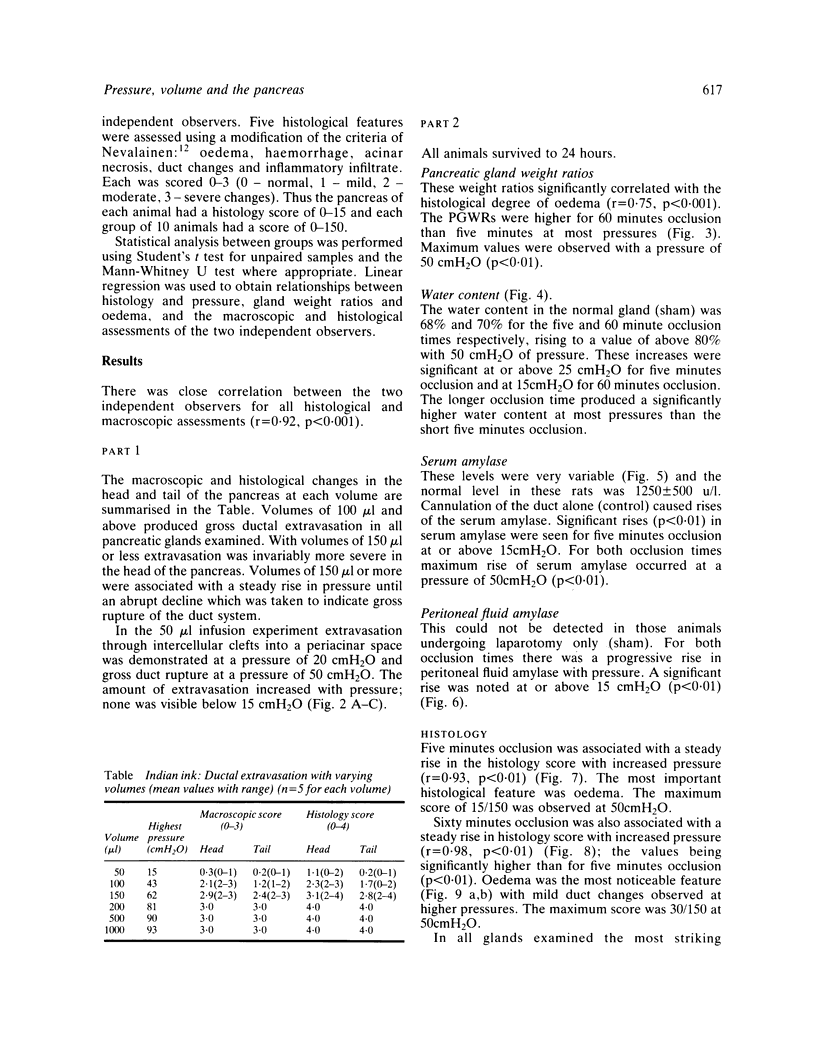
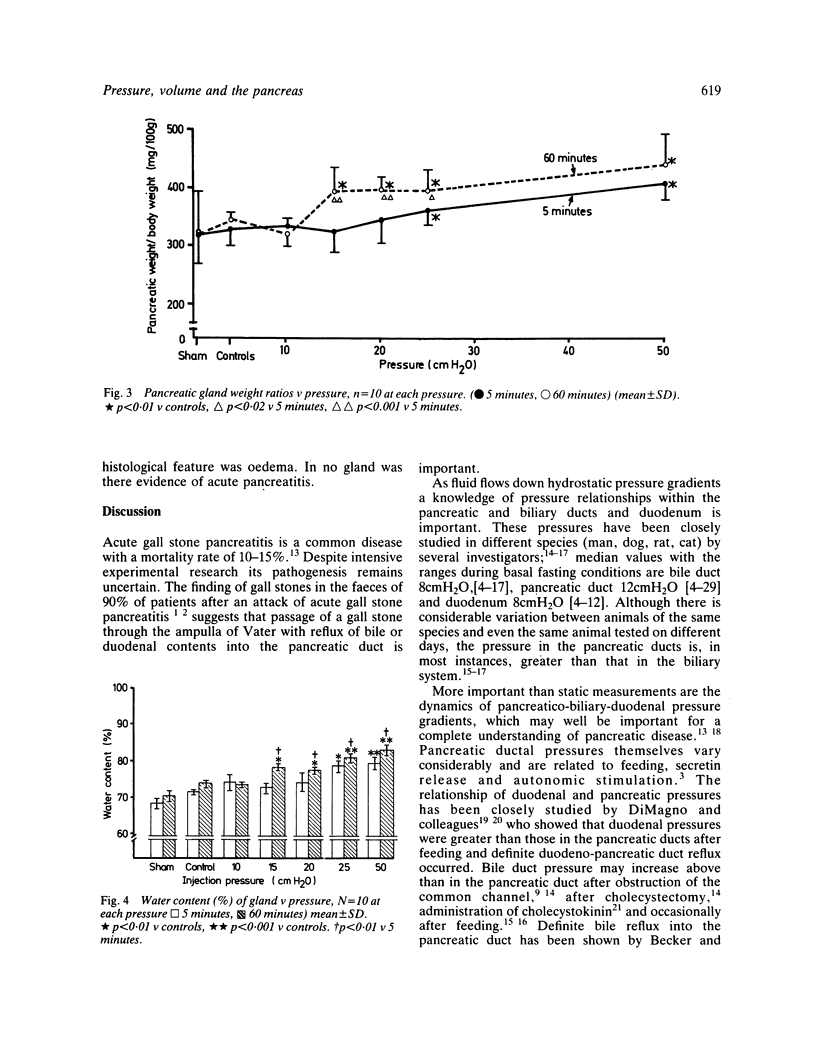
Abstract
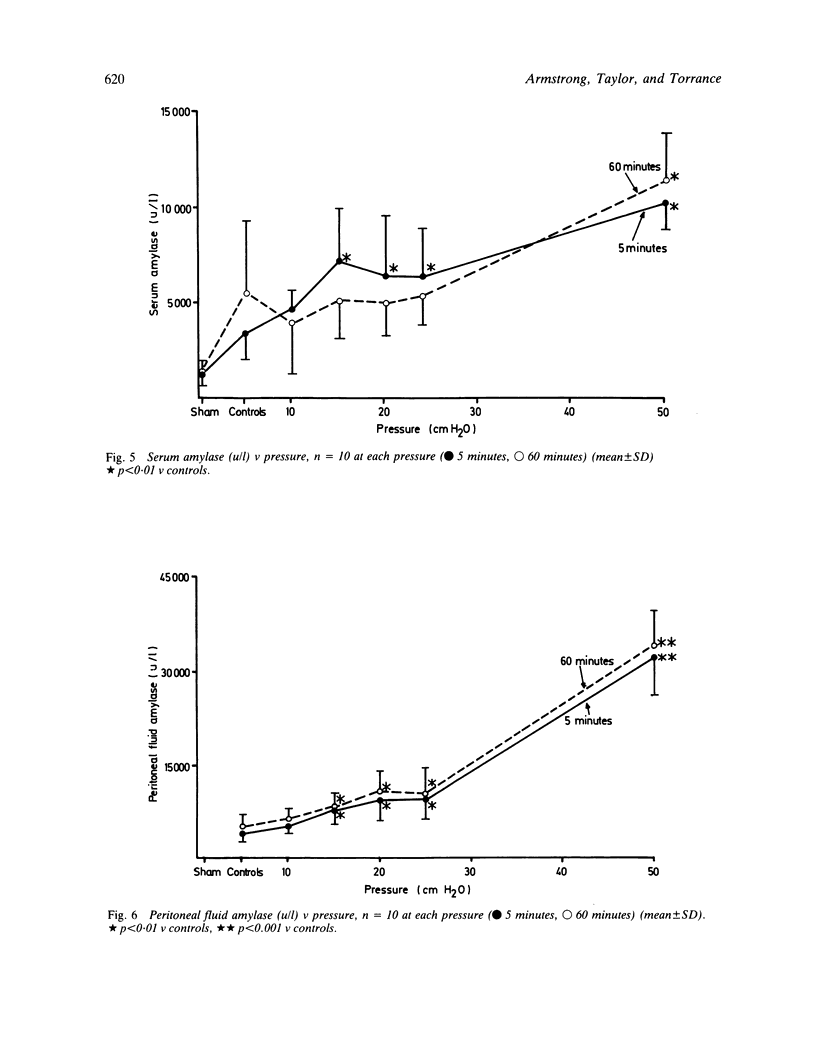
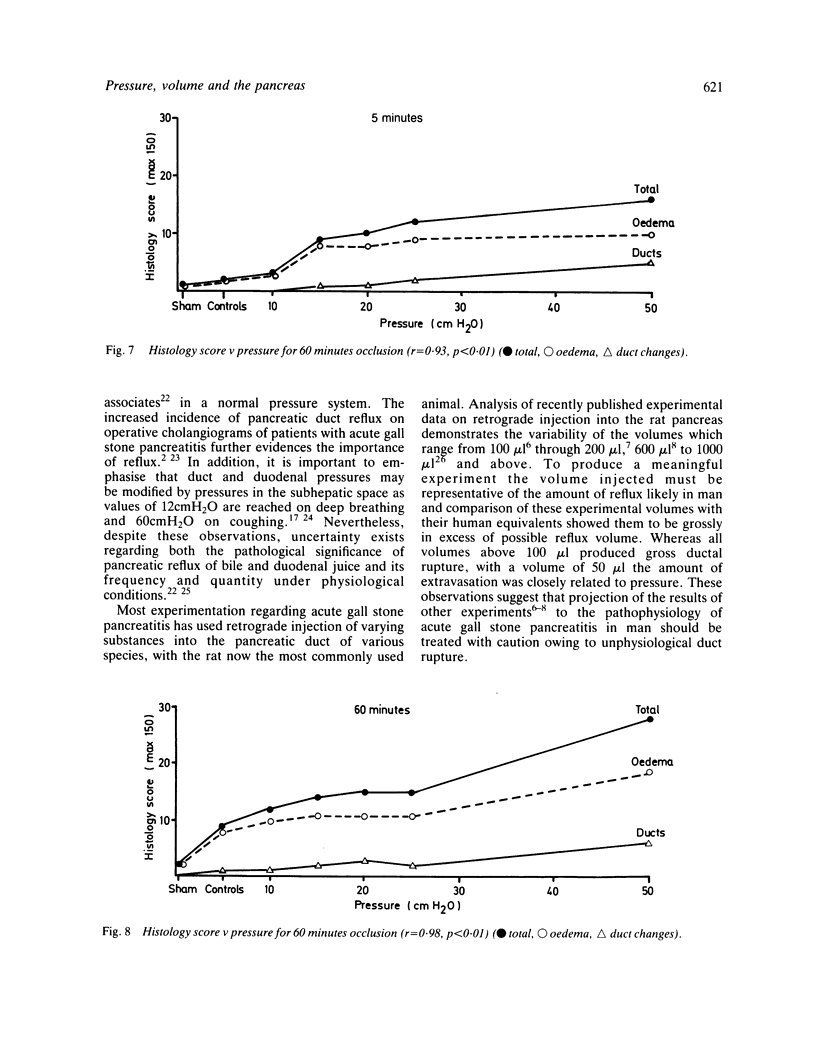
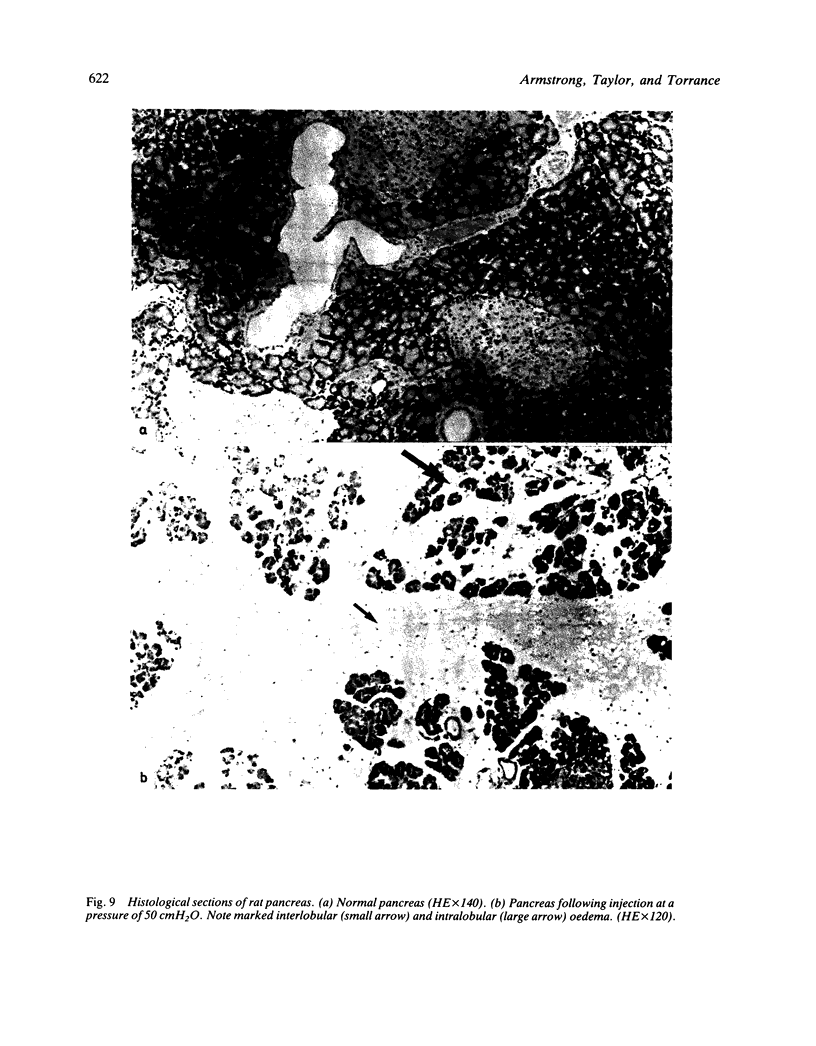
The effects of injection volume and pressure on the rat pancreas have been investigated. An experimental model using transduodenal cannulation of the rat bile-pancreatic duct was used. Injection volumes of 100 microliter or above produced gross ductal extravasation regardless of pressure. With a 50 microliter volume leakage from the ducts occurred via intercellular clefts at a pressure of 20 cmH2O and via duct ruptures at 50 cmH2O. Survival experiments (24 hours) were carried out using the 50 microliter volume. Infusion of 50 microliter saline at increasing pressures produced rises in amylase concentrations, pancreatic gland weights and water content of the gland at pressures of 20 cmH2O or above. These changes were maximal when 50 cmH2O of pressure was maintained for 60 minutes. The changes correlated with extravasation shown by Indian ink. Histological oedema related closely to pressure (r = 0.92), and was the most pronounced histological change observed. In experiments using intraduct injection into the rat pancreas a volume of 50 microliter or less should be used with careful consideration given to pressure. Unless these prerequisites are followed the results of experimental investigation cannot be extrapolated to acute gall stone pancreatitis in man.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta J. M., Ledesma C. L. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974 Feb 28;290(9):484–487. doi: 10.1056/NEJM197402282900904. [DOI] [PubMed] [Google Scholar]
- Aho H. J., Koskensalo S. M., Nevalainen T. J. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15(4):411–416. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
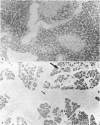
- Aho H. J., Nevalainen T. J., Aho A. J. Experimental pancreatitis in the rat. Development of pancreatic necrosis, ischemia and edema after intraductal sodium taurocholate injection. Eur Surg Res. 1983;15(1):28–36. doi: 10.1159/000128330. [DOI] [PubMed] [Google Scholar]
- Alon U., Berant M., Mordechovitz D., Hashmonai M., Better O. S. Effect of isolated cholaemia on systemic haemodynamics and kidney function in conscious dogs. Clin Sci (Lond) 1982 Jul;63(1):59–64. doi: 10.1042/cs0630059. [DOI] [PubMed] [Google Scholar]
- Anderson M. C., Schiller W. R. Microcirculatory dynamics in the normal and inflamed pancreas. Am J Surg. 1968 Jan;115(1):118–127. doi: 10.1016/0002-9610(68)90139-6. [DOI] [PubMed] [Google Scholar]
- Becker J. M., Ti T. K., Moody F. G., McGreevy J. M., Nelson J. A. A model of biliary pancreatic reflux. Surgery. 1979 Feb;85(2):147–153. [PubMed] [Google Scholar]
- Bockman D. E., Schiller W. R., Anderson M. C. Route of retrograde flow in the exocrine pancreas during ductal hypertension. Arch Surg. 1971 Aug;103(2):321–329. doi: 10.1001/archsurg.1971.01350080237038. [DOI] [PubMed] [Google Scholar]
- Carter D. C. Gall stone pancreatitis. Br Med J (Clin Res Ed) 1983 Apr 23;286(6374):1303–1304. doi: 10.1136/bmj.286.6374.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUPREZ A., GODART S., PLATTEBORSE R., LITVINE J., DUPONT J. M. LA VOIE DE D'ERIVATION INTERSTITIELLE ET LYMPHATIQUE DE LA S'ECR'ETION EXOCRINE DU PANCR'EAS. Bull Acad R Med Belg. 1963;3:691–706. [PubMed] [Google Scholar]
- EGDAHL R. H. Mechanism of blood enzyme changes following the production of experimental pancreatitis. Ann Surg. 1958 Sep;148(3):389–400. doi: 10.1097/00000658-195809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamklou R., Edlund Y. Ductal factors in the pathogenesis of acute pancreatitis in the rat. Scand J Gastroenterol. 1966;1(2):94–100. doi: 10.1080/00365521.1966.11800618. [DOI] [PubMed] [Google Scholar]
- Gedda S., van der Linden W. What makes the peritoneal drain work? Pressure in the subhepatic space after biliary surgery. Acta Chir Scand. 1983;149(7):703–706. [PubMed] [Google Scholar]
- Gilsdorf R. B., Urdaneta L. F., Delaney J. P., Leonard A. S. Central nervous system influences on pancreatic secretion, sphincteric mechanisms, and blood flow and their role in the course of pancreatitis. Surgery. 1967 Oct;62(4):581–588. [PubMed] [Google Scholar]
- Hendricks J. C., DiMagno E. P., Go V. L., Dozois R. R. Reflux of duodenal contents into the pancreatic duct of dogs. J Lab Clin Med. 1980 Nov;96(5):912–921. [PubMed] [Google Scholar]
- Herriott B. A., Palmer A. A. Rupture of small ducts and acini in the pancreas of the rat and guinea pig following major duct obstruction. Aust J Exp Biol Med Sci. 1966 Apr;44(2):143–156. doi: 10.1038/icb.1966.16. [DOI] [PubMed] [Google Scholar]
- Kelly T. R. Gallstone pancreatitis: pathophysiology. Surgery. 1976 Oct;80(4):488–492. [PubMed] [Google Scholar]
- Klein E. S., Grateron H., Rudick J., Dreiling D. A. Pancreatic intraductal pressure: I. A consideration of regulatory factors. Am J Gastroenterol. 1983 Aug;78(8):507–509. [PubMed] [Google Scholar]
- Klein E. S., Grateron H., Toth L., Dreiling D. A. Pancreatic intraductal pressure: II. Effects of autonomic denervation. Am J Gastroenterol. 1983 Aug;78(8):510–512. [PubMed] [Google Scholar]
- Lankisch P. G., Göke B., Fölsch U. R., Winckler K., Otto J., Creutzfeldt W. Influence of secretin on the course of acute experimental pancreatitis in rats. Digestion. 1983;26(4):187–191. doi: 10.1159/000198888. [DOI] [PubMed] [Google Scholar]
- MENGUY R. B., HALLENBECK G. A., BOLLMAN J. L., GRINDLAY J. H. Intraductal pressures and sphincteric resistance in canine pancreatic and biliary ducts after various stimuli. Surg Gynecol Obstet. 1958 Mar;106(3):306–320. [PubMed] [Google Scholar]
- McCutcheon A. D. A fresh approach to the pathogenesis of pancreatitis. Gut. 1968 Jun;9(3):296–310. doi: 10.1136/gut.9.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen T. J., Seppä A. Acute pancreatitis caused by closed duodenal loop in the rat. Scand J Gastroenterol. 1975;10(5):521–527. [PubMed] [Google Scholar]
- Owyang C., Dozois R. R., DiMagno E. P., Go V. L. Relationships between fasting and postprandial pancreaticoduodenal pressures, pancreatic secretion, and duodenal volume flow in the dog. Gastroenterology. 1977 Nov;73(5):1046–1049. [PubMed] [Google Scholar]
- PARRY E. W., HALLENBECK G. A., GRINDLAY J. H. Pressures in the pancreatic and common ducts; values during fasting, after various meals, and after sphincterotomy; an experimental study. AMA Arch Surg. 1955 May;70(5):757–765. doi: 10.1001/archsurg.1955.01270110129018. [DOI] [PubMed] [Google Scholar]
- Pirola R. C., Davis A. E. Effect of pressure on the integrity of the duct-acinar system of the pancreas. Gut. 1970 Jan;11(1):69–73. doi: 10.1136/gut.11.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncelet P., Thompson A. G. Action of bile phospholipids on the pancreas. Am J Surg. 1972 Feb;123(2):196–202. doi: 10.1016/0002-9610(72)90333-9. [DOI] [PubMed] [Google Scholar]
- RICHARDS C., FITZGERALD P. J., CAROL B., ROSENSTOCK L., LIPKIN L. SEGMENTAL DIVISION OF THE RAT PANCREAS FOR EXPERIMENTAL PROCEDURES. Lab Invest. 1964 Oct;13:1303–1321. [PubMed] [Google Scholar]
- ROBINSON T. M., DUNPHY J. E. Continuous perfusion of bile and protease activators through the pancreas. JAMA. 1963 Feb 16;183:530–533. doi: 10.1001/jama.1963.63700070004009a. [DOI] [PubMed] [Google Scholar]
- Reber H. A., Roberts C., Way L. W. The pancreatic duct mucosal barrier. Am J Surg. 1979 Jan;137(1):128–134. doi: 10.1016/0002-9610(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Taylor T. V., Rimmer S. Pancreatic-duct reflux in patients with gallstone pancreatitis? Lancet. 1980 Apr 19;1(8173):848–850. doi: 10.1016/s0140-6736(80)91354-9. [DOI] [PubMed] [Google Scholar]