Abstract
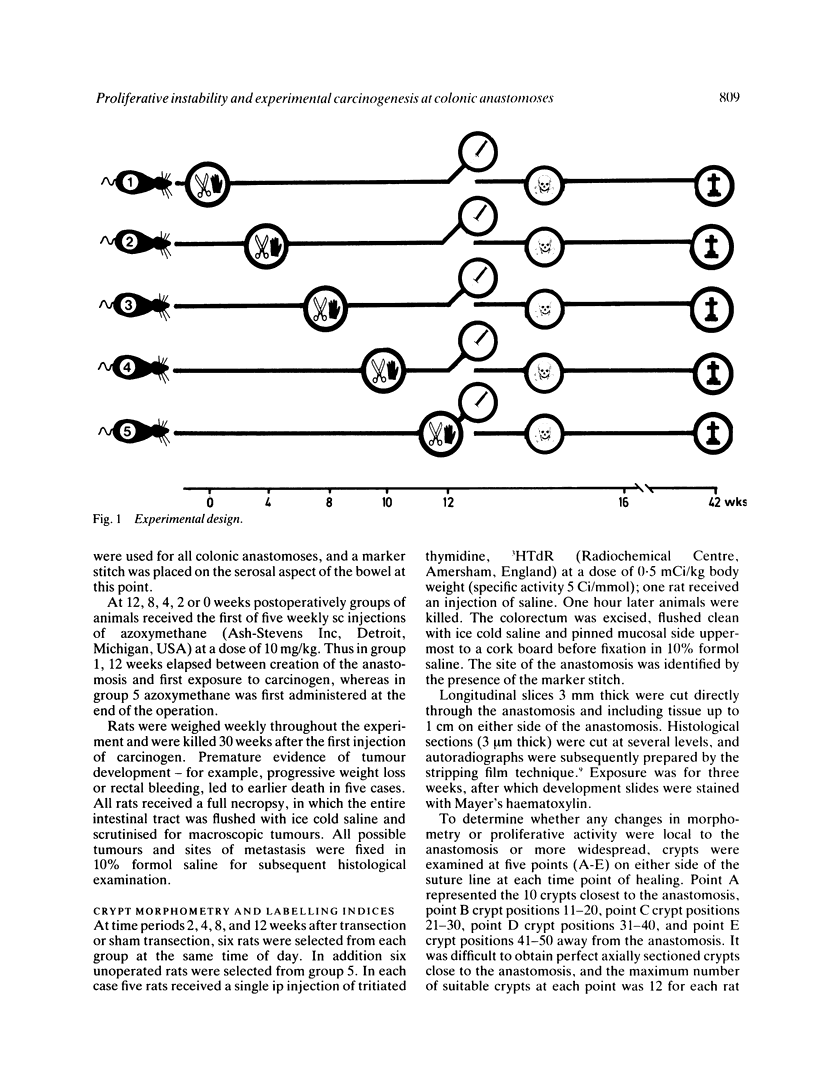
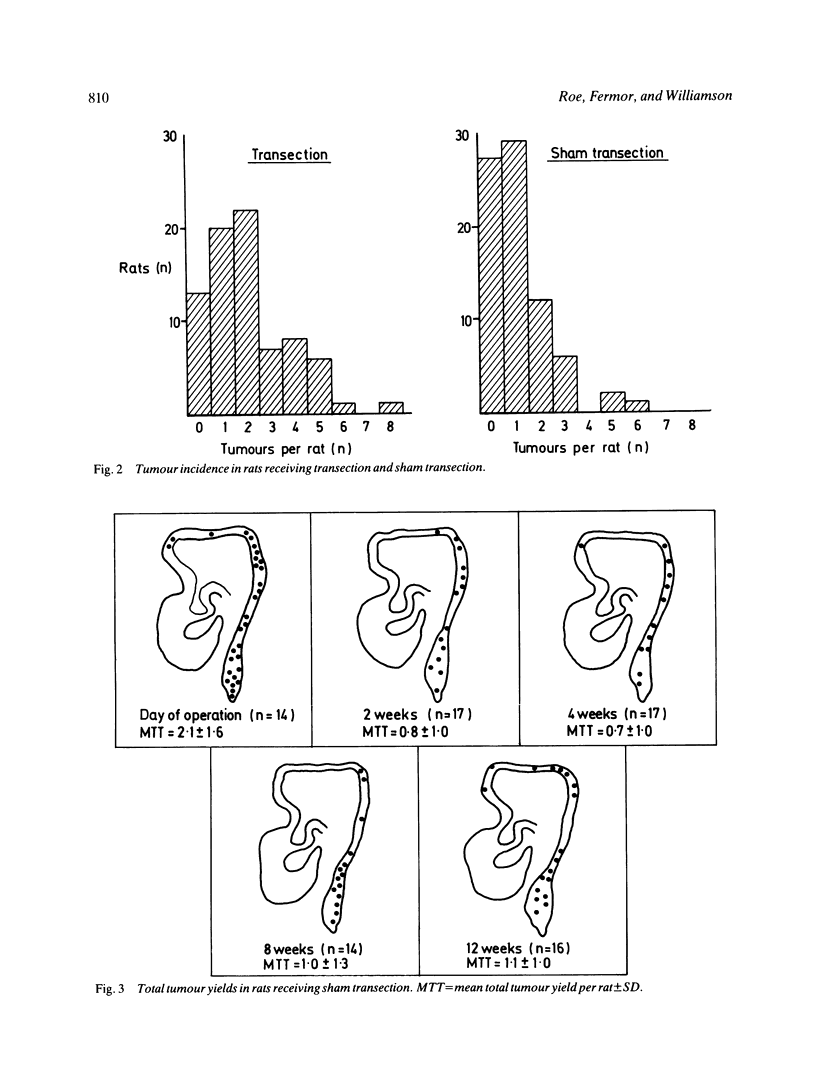
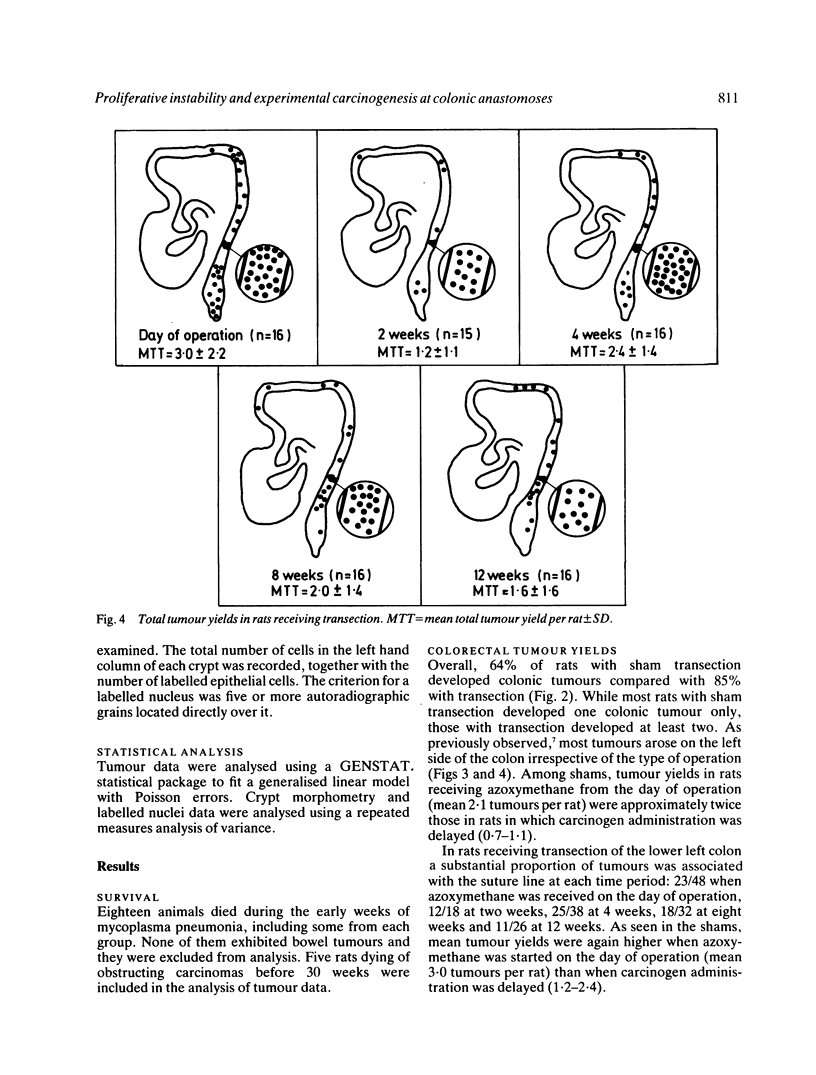
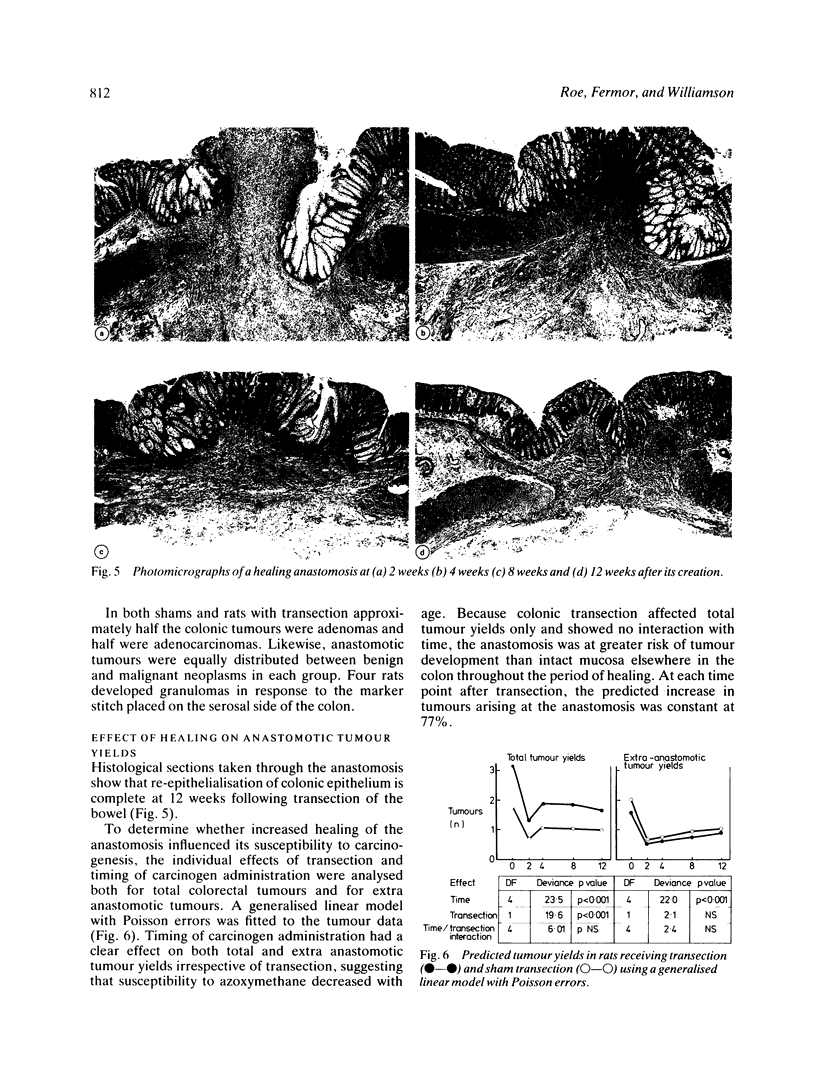
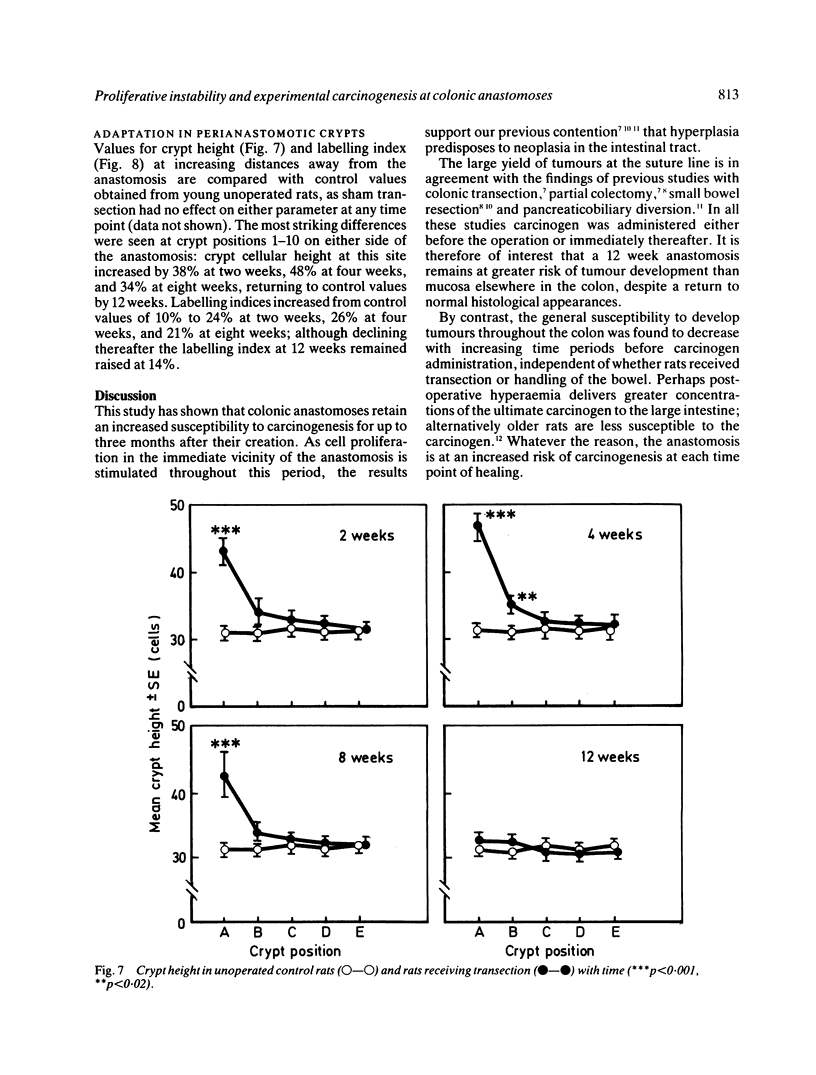
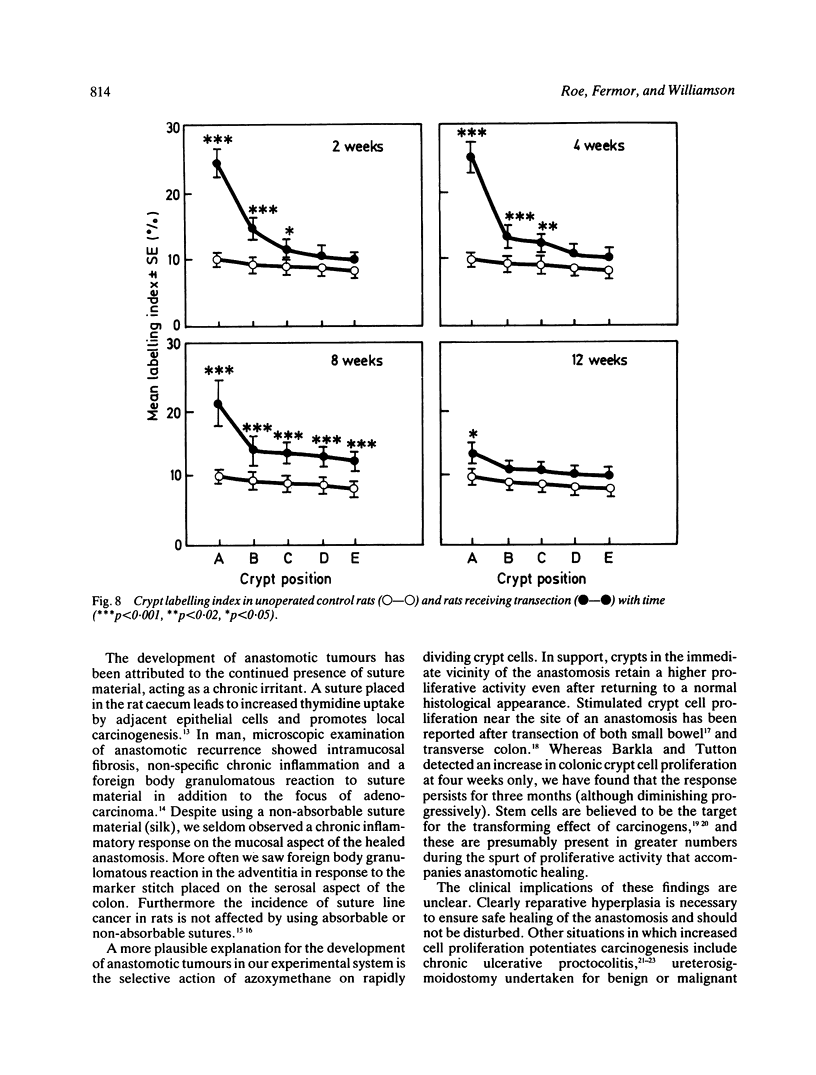
The possibility that proliferative instability around a healing anastomosis promotes carcinogenesis was tested in 234 male Sprague-Dawley rats. Animals received the first of five weekly injections of azoxymethane (total dose 50 mg/kg) either immediately after transection of the descending colon or at 2, 4, 8, and 12 weeks later; controls received handling of the bowel alone. Crypt cell proliferation was assessed by autoradiography following 3HTdR injection. An overall increase in tumour yields in all transection groups was due solely to the frequent presence of anastomotic tumours. Changes in crypt morphometry and labelling index were most marked in crypt positions 1-10 away from the anastomosis. Crypts at this site increased in height at 2, 4, and 8 weeks (p less than 0.001) but returned to normal values by 12 weeks. Likewise, labelling index was increased at 2, 4, and 8 weeks (p less than 0.001) and remained higher at 12 weeks (p less than 0.05). Increased crypt cell proliferation in the immediate vicinity of an apparently 'healed' colonic anastomosis may explain its persisting susceptibility to carcinogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan A., Bristol J. B., Williamson R. C. Crypt cell production rate in ulcerative proctocolitis: differential increments in remission and relapse. Gut. 1985 Oct;26(10):999–1003. doi: 10.1136/gut.26.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. M. Chemoradiotherapeutic prevention of local recurrence after stapled anastomoses in rectal cancer. Scott Med J. 1981 Jan;26(1):21–23. doi: 10.1177/003693308102600106. [DOI] [PubMed] [Google Scholar]
- Barkla D. H., Tutton P. M. The influence of surgical transection and anastomosis on the rate of cell proliferation in the colonic epithelium of normal and DMH-treated rats. Carcinogenesis. 1983 Oct;4(10):1323–1325. doi: 10.1093/carcin/4.10.1323. [DOI] [PubMed] [Google Scholar]
- Bykorez A. I., Ivashchenko YuD Gastrointestinal stem cells and their role in carcinogenesis. Int Rev Cytol. 1984;90:309–373. doi: 10.1016/s0074-7696(08)61493-x. [DOI] [PubMed] [Google Scholar]
- Byrne P. J., Stephens R. B., Hennessy T. P., West A. B., Sheahan D. G. Colon cancer at sites of anastomosis. Lancet. 1984 Jan 28;1(8370):225–225. doi: 10.1016/s0140-6736(84)92148-2. [DOI] [PubMed] [Google Scholar]
- Calderisi R. N., Freeman H. J. Differential effects of surgical suture materials in 1,2-dimethylhydrazine-induced rat intestinal neoplasia. Cancer Res. 1984 Jul;44(7):2827–2830. [PubMed] [Google Scholar]
- Chester J. F., Gaissert H. A., Ross J. S., Malt R. A., Weitzman S. A. Augmentation of 1,2-dimethylhydrazine-induced colon cancer by experimental colitis in mice: role of dietary vitamin E. J Natl Cancer Inst. 1986 May;76(5):939–942. [PubMed] [Google Scholar]
- Druckrey H., Lange A. Carcinogenicity of azoxymethane dependent on age in BD rats. Fed Proc. 1972 Sep-Oct;31(5):1482–1484. [PubMed] [Google Scholar]
- Enker W. E., Dragacevic S. Multiple carcinomas of the large bowel: a natural experiment in etiology and pathogenesis. Ann Surg. 1978 Jan;187(1):8–11. doi: 10.1097/00000658-197801000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte P. J., Steele G., Jr, Rayner A. A., Munroe A. E., King V. P., Wilson R. E. Effect of major small bowel resection on dimethylhydrazine-induced bowel carcinogenesis. J Surg Oncol. 1981;18(1):87–93. doi: 10.1002/jso.2930180113. [DOI] [PubMed] [Google Scholar]
- Hickey R. C., Romsdahl M. M., Johnson D. E., Wallace S., Mavligit G. M., Leavens M. E., Borgelt B. Recurrent cancer and metastases. World J Surg. 1982 Sep;6(5):585–595. doi: 10.1007/BF01657873. [DOI] [PubMed] [Google Scholar]
- Maratka Z., Nedbal J., Kociánová J., Havelka J., Kudrmann J., Hendl J. Incidence of colorectal cancer in proctocolitis: a retrospective study of 959 cases over 40 years. Gut. 1985 Jan;26(1):43–49. doi: 10.1136/gut.26.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozharisski K. M. The significance of nonspecific injury for colon carcinogenesis in rats. Cancer Res. 1975 Dec;35(12):3824–3830. [PubMed] [Google Scholar]
- Sandler R. S., Sandler D. P. Radiation-induced cancers of the colon and rectum: assessing the risk. Gastroenterology. 1983 Jan;84(1):51–57. [PubMed] [Google Scholar]
- Sharp J. G., Hanson W. R., Osborne J. W. The relationship between the compensatory response in the stomach and colon and the extent of small bowel resection in the rat. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;44(3):295–304. doi: 10.1007/BF02890179. [DOI] [PubMed] [Google Scholar]
- Stewart M. Urinary diversion and bowel cancer. Ann R Coll Surg Engl. 1986 Mar;68(2):98–102. [PMC free article] [PubMed] [Google Scholar]
- Umpleby H. C., Bristol J. B., Rainey J. B., Williamson R. C. Survival of 727 patients with single carcinomas of the large bowel. Dis Colon Rectum. 1984 Dec;27(12):803–810. doi: 10.1007/BF02553944. [DOI] [PubMed] [Google Scholar]
- Umpleby H. C., Fermor B., Symes M. O., Williamson R. C. Viability of exfoliated colorectal carcinoma cells. Br J Surg. 1984 Sep;71(9):659–663. doi: 10.1002/bjs.1800710902. [DOI] [PubMed] [Google Scholar]
- Welch J. P., Donaldson G. A. The clinical correlation of an autopsy study of recurrent colorectal cancer. Ann Surg. 1979 Apr;189(4):496–502. doi: 10.1097/00000658-197904000-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Oscarson J. E., Ross J. S., Malt R. A. Promotion of azoxymethane-induced colonic neoplasia by resection of the proximal small bowel. Cancer Res. 1978 Oct;38(10):3212–3217. [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Ross J. S., Watkins J. B., Malt R. A. Enhanced colonic carcinogenesis with azoxymethane in rats after pancreaticobiliary diversion to mid small bowel. Gastroenterology. 1979 Jun;76(6):1386–1392. [PubMed] [Google Scholar]
- Williamson R. C., Davies P. W., Bristol J. B., Wells M. Intestinal adaptation and experimental carcinogenesis after partial colectomy. Increased tumour yields are confined to the anastomosis. Gut. 1982 Apr;23(4):316–325. doi: 10.1136/gut.23.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]



