Abstract
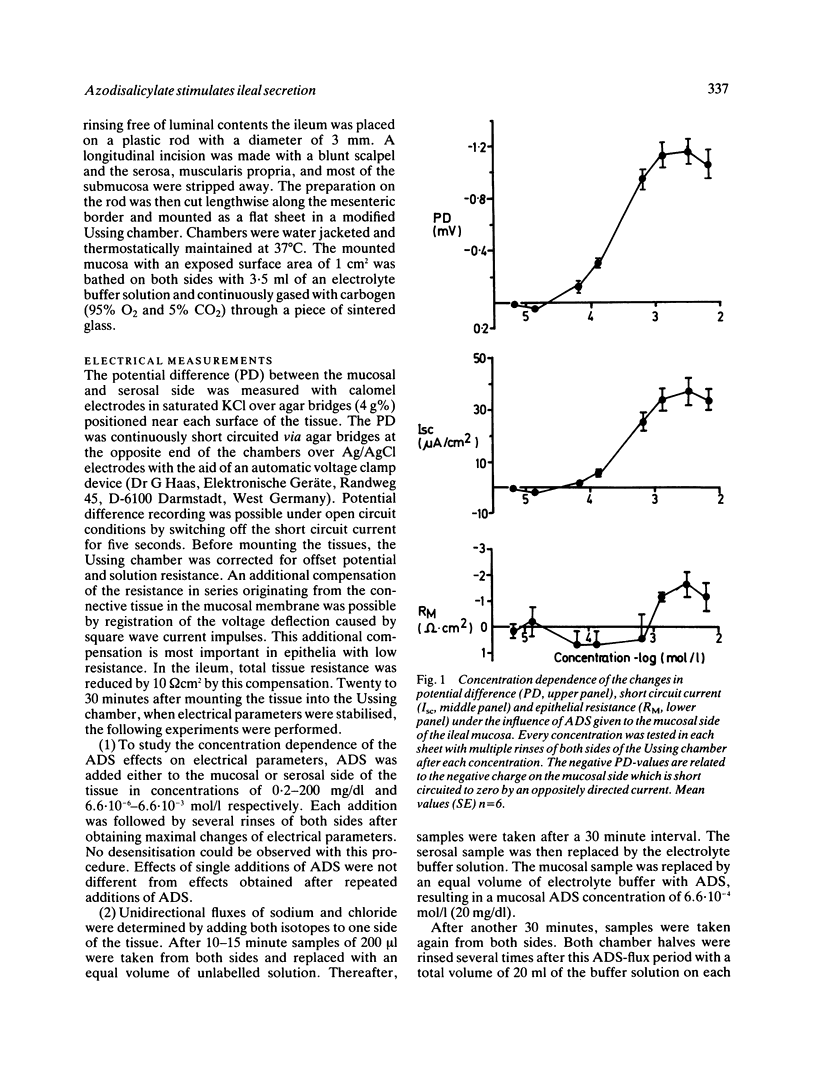
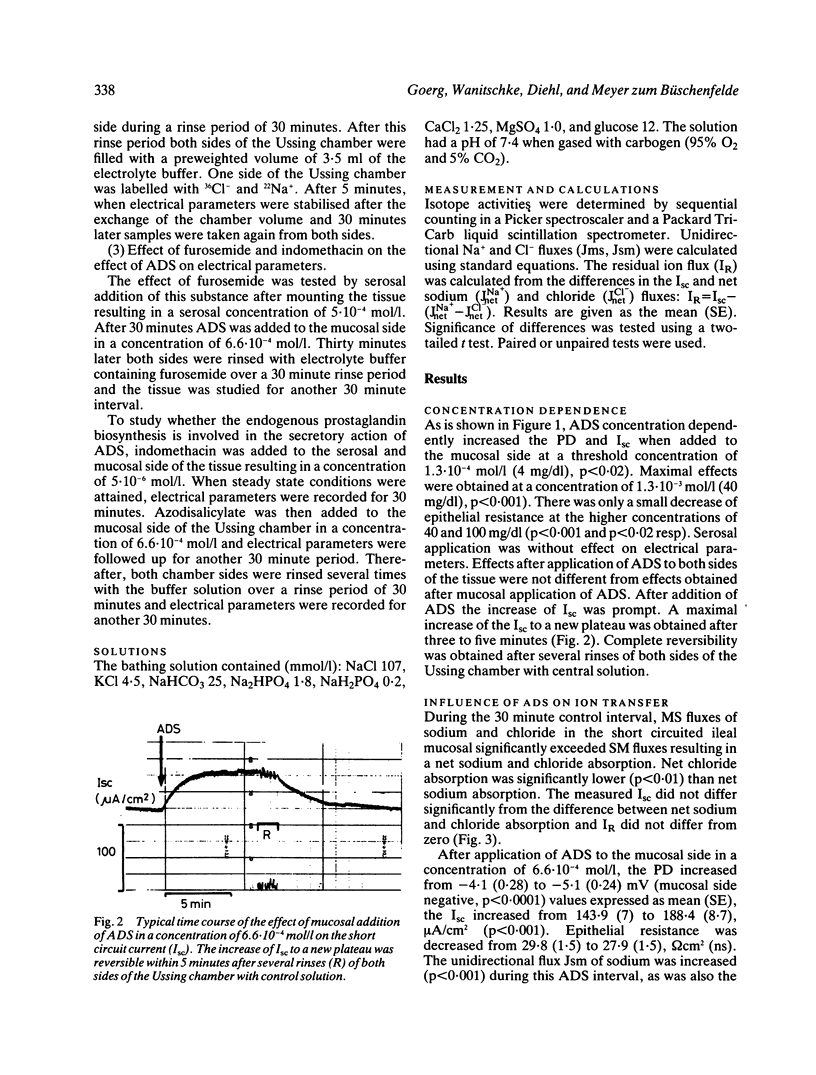
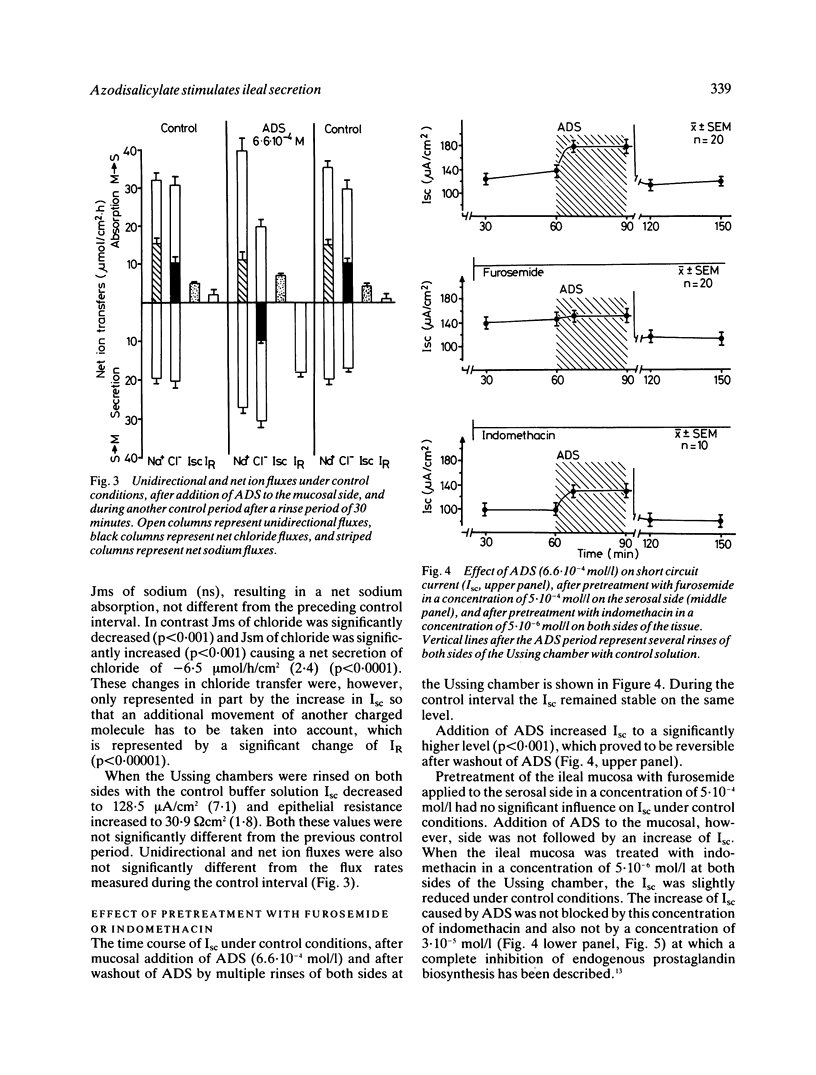
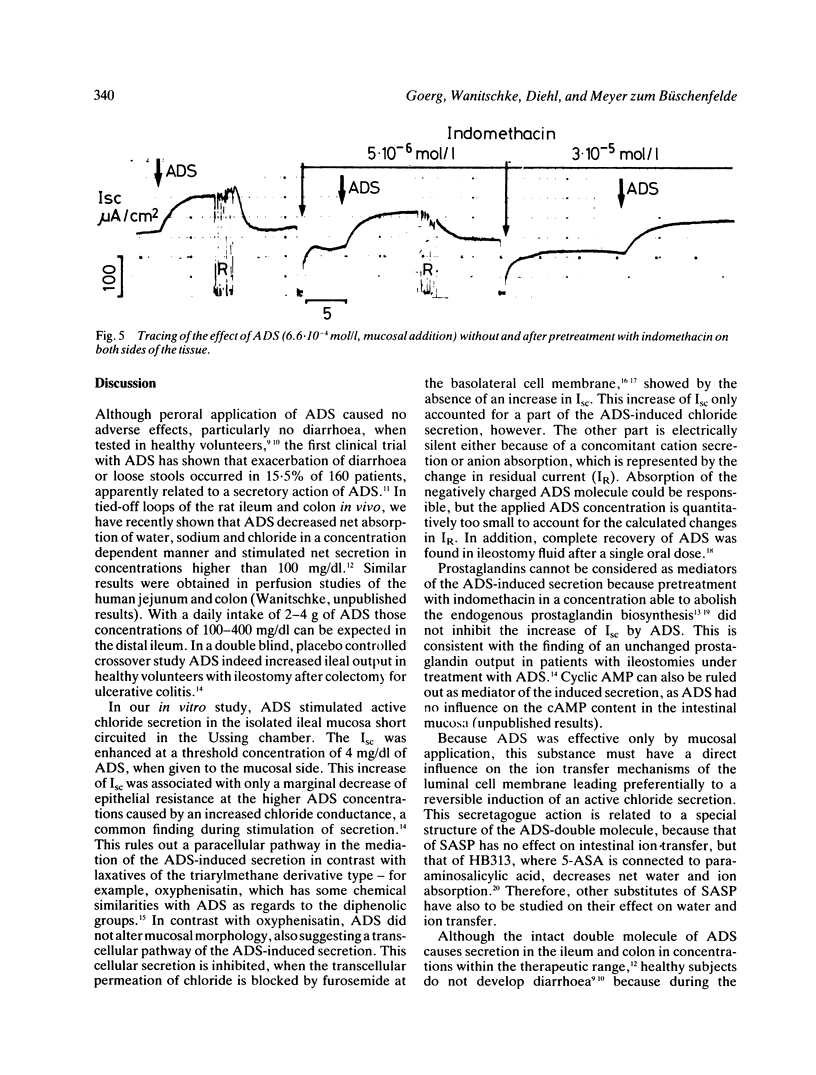
Azodisalicylate (ADS) is one of the newly developed substitutes of sulphasalazine consisting of two molecules of 5-amino-salicylic acid. Azodisalicylate caused diarrhoea in some patients, apparently caused by an antiabsorptive secretagogue action of this compound. The mechanism of this was studied in the short circuited isolated mucosa of the rat ileum. Mucosal addition of ADS increased the potential difference (PD) and short circuit current (Isc) at a concentration of 1.3.10(-4) mol/l (4 mg/dl) with maximal effects at 1.3.10(-3) mol/l (40 mg/dl). Epithelial resistance was only slightly decreased at the higher concentrations of 40 and 100 mg/dl. Serosal ADS had no effect on electrical parameters. The increase of Isc was associated with a change of net chloride absorption into net secretion. Net sodium absorption was only slightly and not significantly decreased. The changes were reversible after rinsing away the ADS. Treatment of the mucosa with furosemide inhibited the ADS induced increase of Isc, suggesting transcellular pathway for the ADS stimulated secretion. Biosynthesis of prostaglandins is not involved in the mechanism of this secretion, as treatment with indomethacin did not alter the effect of ADS on Isc. Results suggest that ADS can be considered as a secretagogue, which stimulates intestinal secretion via a transcellular pathway. Because of the bacterial cleavage of the double molecule into two molecules of the non-secretagogue 5-amino-salicylic acid in the colon, however, diarrhoea may develop only in patients with a decreased absorptive capacity of the colon, or insufficient cleavage of ADS.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azad Khan A. K., Piris J., Truelove S. C. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet. 1977 Oct 29;2(8044):892–895. doi: 10.1016/s0140-6736(77)90831-5. [DOI] [PubMed] [Google Scholar]
- Bukhave K., Rask-Madsen J. Saturation kinetics applied to in vitro effects of low prostaglandin E2 and F 2 alpha concentrations on ion transport across human jejunal mucosa. Gastroenterology. 1980 Jan;78(1):32–42. [PubMed] [Google Scholar]
- Das K. M., Eastwood M. A., McManus J. P., Sircus W. Adverse reactions during salicylazosulfapyridine therapy and the relation with drug metabolism and acetylator phenotype. N Engl J Med. 1973 Sep 6;289(10):491–495. doi: 10.1056/NEJM197309062891001. [DOI] [PubMed] [Google Scholar]
- Debongnie J. C., Phillips S. F. Capacity of the human colon to absorb fluid. Gastroenterology. 1978 Apr;74(4):698–703. [PubMed] [Google Scholar]
- Goldman P. Will there be a next generation of sulfasalazine? Gastroenterology. 1982 Nov;83(5):1138–1141. [PubMed] [Google Scholar]
- Heintze K., Stewart C. P., Frizzell R. A. Sodium-dependent chloride secretion across rabbit descending colon. Am J Physiol. 1983 Apr;244(4):G357–G365. doi: 10.1152/ajpgi.1983.244.4.G357. [DOI] [PubMed] [Google Scholar]
- Klotz U., Maier K., Fischer C., Heinkel K. Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn's disease. N Engl J Med. 1980 Dec 25;303(26):1499–1502. doi: 10.1056/NEJM198012253032602. [DOI] [PubMed] [Google Scholar]
- Lauritsen K., Hansen J., Ryde M., Rask-Madsen J. Colonic azodisalicylate metabolism determined by in vivo dialysis in healthy volunteers and patients with ulcerative colitis. Gastroenterology. 1984 Jun;86(6):1496–1500. [PubMed] [Google Scholar]
- Peppercorn M. A., Goldman P. Distribution studies of salicylazosulfapyridine and its metabolites. Gastroenterology. 1973 Feb;64(2):240–245. [PubMed] [Google Scholar]
- Sandberg-Gertzén H., Järnerot G., Bukhave K., Lauritsen K., Rask-Madsen J. Effect of azodisal sodium and sulphasalazine on ileostomy output of fluid and PGE2 and PGF2 alpha in subjects with a permanent ileostomy. Gut. 1986 Nov;27(11):1306–1311. doi: 10.1136/gut.27.11.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg-Gertzén H., Järnerot G., Kraaz W. Azodisal sodium in the treatment of ulcerative colitis. A study of tolerance and relapse-prevention properties. Gastroenterology. 1986 Apr;90(4):1024–1030. doi: 10.1016/0016-5085(86)90882-6. [DOI] [PubMed] [Google Scholar]
- Sandberg-Gertzén H., Ryde M., Järnerot G. Absorption and excretion of a single 1-g dose of azodisal sodium in subjects with ileostomy. Scand J Gastroenterol. 1983 Jan;18(1):107–111. doi: 10.3109/00365528309181568. [DOI] [PubMed] [Google Scholar]
- Schröder H., Campbell D. E. Absorption, metabolism, and excretion of salicylazosulfapyridine in man. Clin Pharmacol Ther. 1972 Jul-Aug;13(4):539–551. doi: 10.1002/cpt1972134539. [DOI] [PubMed] [Google Scholar]
- Taffet S. L., Das K. M. Desensitization of patients with inflammatory bowel disease to sulfasalazine. Am J Med. 1982 Oct;73(4):520–524. doi: 10.1016/0002-9343(82)90330-8. [DOI] [PubMed] [Google Scholar]
- Willoughby C. P., Aronson J. K., Agback H., Bodin N. O., Truelove S. C. Distribution and metabolism in healthy volunteers of disodium azodisalicylate, a potential therapeutic agent for ulcerative colitis. Gut. 1982 Dec;23(12):1081–1087. doi: 10.1136/gut.23.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees P. A., Bakker J. H., van Tongeren J. H. Effect of sulphapyridine, 5-aminosalicylic acid, and placebo in patients with idiopathic proctitis: a study to determine the active therapeutic moiety of sulphasalazine. Gut. 1980 Jul;21(7):632–635. doi: 10.1136/gut.21.7.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees P. A., Tuinte J. H., van Rossum J. M., van Tongeren J. H. Influence of intestinal transit time on azo-reduction of salicylazosulphapyridine (Salazopyrin). Gut. 1979 Apr;20(4):300–304. doi: 10.1136/gut.20.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hogezand R. A., van Hees P. A., Zwanenburg B., van Rossum J. M., van Tongeren J. H. Disposition of disodium azodisalicylate in healthy subjects. A possible new drug for inflammatory bowel disease. Gastroenterology. 1985 Mar;88(3):717–722. doi: 10.1016/0016-5085(85)90142-8. [DOI] [PubMed] [Google Scholar]


