Abstract
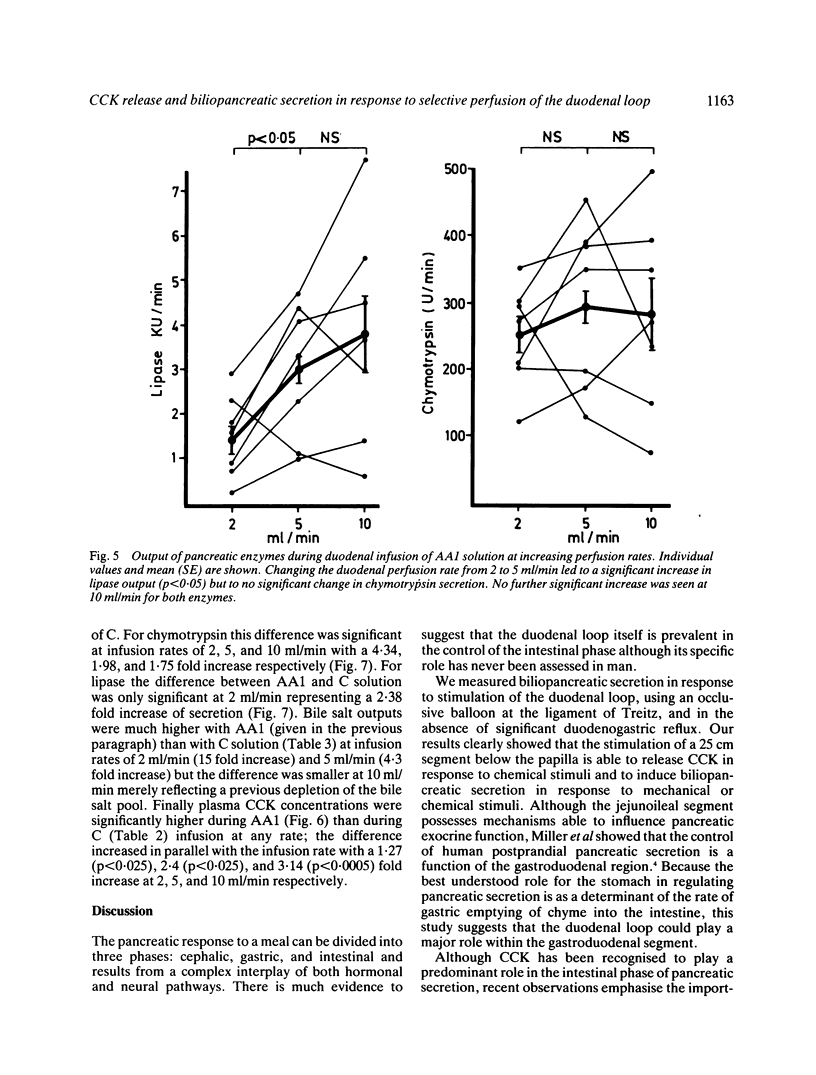
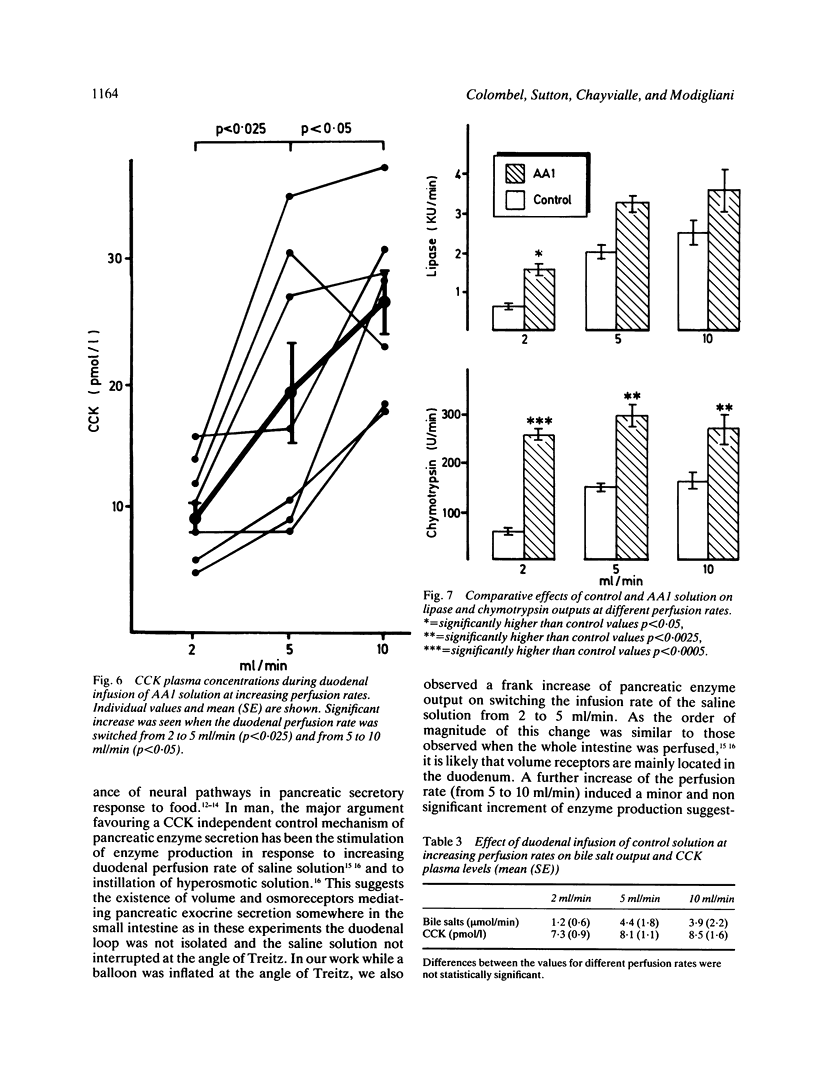
The aim of this study was to measure the role of the duodenal loop in biliopancreatic secretion in man by infusing various stimuli at the ampulla of Vater and collecting duodenal contents at the ligament of Treitz, above an occluding balloon. Perfusion at 10 ml/min of a first mixture of aminoacids - phenylalanine (47.2 mmol), methionine (38.2 mmol), tryptophan (11 mmol), valine (61.6 mmol) - increased cholecystokinin (CCK) plasma concentrations and duodenal bile salt output (p less than 0.005) as compared with a control electrolyte solution, but did not change pancreatic enzyme secretion significantly; duodenal infusion of another aminoacid mixture - arginine (32.4 mmol), histidine (14.1 mmol), leucine (36 mmol), isoleucine (21.5 mmol), lysine (31 mmol), threonine (23 mmol) - did not change CCK plasma concentrations, bile salt or pancreatic enzyme output. The respective role of duodenal distension and endogenous CCK was investigated by perfusing the first aminoacid solution and the control solution at 2, 5, and 10 ml/min. Changing the perfusion rate of control solution from 2 to 5 ml/min led to a significant increase (p less than 0.01) in pancreatic secretion with no further increase at 10 ml/min. Bile salt output was not influenced by the perfusion rate of control solution. During the perfusion of the aminoacid solution, despite a stepwise increase in CCK release, the only significant change in pancreatic secretion was an increase of lipase output (p less than 0.05) when the infusion rate was raised from 2 to 5 ml/min. Our results suggest that duodenal CCK release (1) depends on the nature of aminoacids (2) has predominant role in the regulation of pancreatic secretion at low perfusion rate but is less effective when superimposed on a mechanical stimulus caused by duodenal distension (3) is a major stimulus for gall bladder contraction which is not influenced by duodenal distension.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge W. R., Burke C. A., Izzo R. S., Praissman M. Role of cholecystokinin in intestinal phase of human pancreatic secretion. Dig Dis Sci. 1987 Feb;32(2):155–163. doi: 10.1007/BF01297103. [DOI] [PubMed] [Google Scholar]
- Dooley C. P., Valenzuela J. E. Duodenal volume and osmoreceptors in the stimulation of human pancreatic secretion. Gastroenterology. 1984 Jan;86(1):23–27. [PubMed] [Google Scholar]
- FIGARELLA C., TAULIER J., SARLES H. DOSAGE DE LA CHYMOTRYPSINE ET DE LA TRYPSINE DANS LE SUC DUOD'ENAL. Bull Soc Chim Biol (Paris) 1965;47:679–686. [PubMed] [Google Scholar]
- Fisher R. S., Rock E., Malmud L. S. Cholinergic effects on gallbladder emptying in humans. Gastroenterology. 1985 Oct;89(4):716–722. doi: 10.1016/0016-5085(85)90564-5. [DOI] [PubMed] [Google Scholar]
- Go V. L., Hofmann A. F., Summerskill W. H. Pancreozymin bioassay in man based on pancreatic enzyme secretion: potency of specific amino acids and other digestive products. J Clin Invest. 1970 Aug;49(8):1558–1564. doi: 10.1172/JCI106373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedon C., Ducrotte P., Chayvialle J. A., Lerebours E., Denis P., Colin R. Effects of intravenous and intraduodenal fat on jejunal motility and on plasma cholecystokinin in man. Dig Dis Sci. 1988 May;33(5):558–564. doi: 10.1007/BF01798357. [DOI] [PubMed] [Google Scholar]
- Izzo R. S., Brugge W. R., Praissman M. Immunoreactive cholecystokinin in human and rat plasma: correlation of pancreatic secretion in response to CCK. Regul Pept. 1984 Sep;9(1-2):21–34. doi: 10.1016/0167-0115(84)90004-1. [DOI] [PubMed] [Google Scholar]
- MARCHIS-MOUREN G., SARDA L., DESNUELLE P. Purification of hog pancreatic lipase. Arch Biochem Biophys. 1959 Jul;83(1):309–319. doi: 10.1016/0003-9861(59)90036-0. [DOI] [PubMed] [Google Scholar]
- Meyer J. H., Kelly G. A., Spingola L. J., Jones R. S. Canine gut receptors mediating pancreatic responses to luminal L-amino acids. Am J Physiol. 1976 Sep;231(3):669–677. doi: 10.1152/ajplegacy.1976.231.3.669. [DOI] [PubMed] [Google Scholar]
- Miller L. J., Clain J. E., Malagelada J. R., Go V. L. Control of human postprandial pancreatic exocrine secretion: a function of the gastroduodenal region. Dig Dis Sci. 1979 Feb;24(2):150–154. doi: 10.1007/BF01324743. [DOI] [PubMed] [Google Scholar]
- Murphy G. M., Billing B. H., Baron D. N. A fluorimetric and enzymatic method for the estimation of serum total bile acids. J Clin Pathol. 1970 Oct;23(7):594–598. doi: 10.1136/jcp.23.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owyang C., May D., Louie D. S. Trypsin suppression of pancreatic enzyme secretion. Differential effect on cholecystokinin release and the enteropancreatic reflex. Gastroenterology. 1986 Sep;91(3):637–643. doi: 10.1016/0016-5085(86)90633-5. [DOI] [PubMed] [Google Scholar]
- Singer M. V. Réponse sécrétoire pancréatique aux stimulants intestinaux. Gastroenterol Clin Biol. 1986 Jun-Jul;10(6-7):504–512. [PubMed] [Google Scholar]
- Solomon T. E., Grossman M. I. Effect of atropine and vagotomy on response of transplanted pancreas. Am J Physiol. 1979 Feb;236(2):E186–E190. doi: 10.1152/ajpendo.1979.236.2.E186. [DOI] [PubMed] [Google Scholar]
- Solomon T. E. Regulation of pancreatic secretion. Clin Gastroenterol. 1984 Sep;13(3):657–678. [PubMed] [Google Scholar]
- Thomas F. B., Sinar D., Mazzaferri E. L., Cataland S., Mekhjian H. S., Caldwell J. H., Fromkes J. J. Selective release of gastric inhibitory polypeptide by intraduodenal amino acid perfusion in man. Gastroenterology. 1978 Jun;74(6):1261–1265. [PubMed] [Google Scholar]
- Watanabe S., Shiratori K., Takeuchi T., Chey W. Y., You C. H., Chang T. M. Release of cholecystokinin and exocrine pancreatic secretion in response to an elemental diet in human subjects. Dig Dis Sci. 1986 Sep;31(9):919–924. doi: 10.1007/BF01303211. [DOI] [PubMed] [Google Scholar]


