Abstract
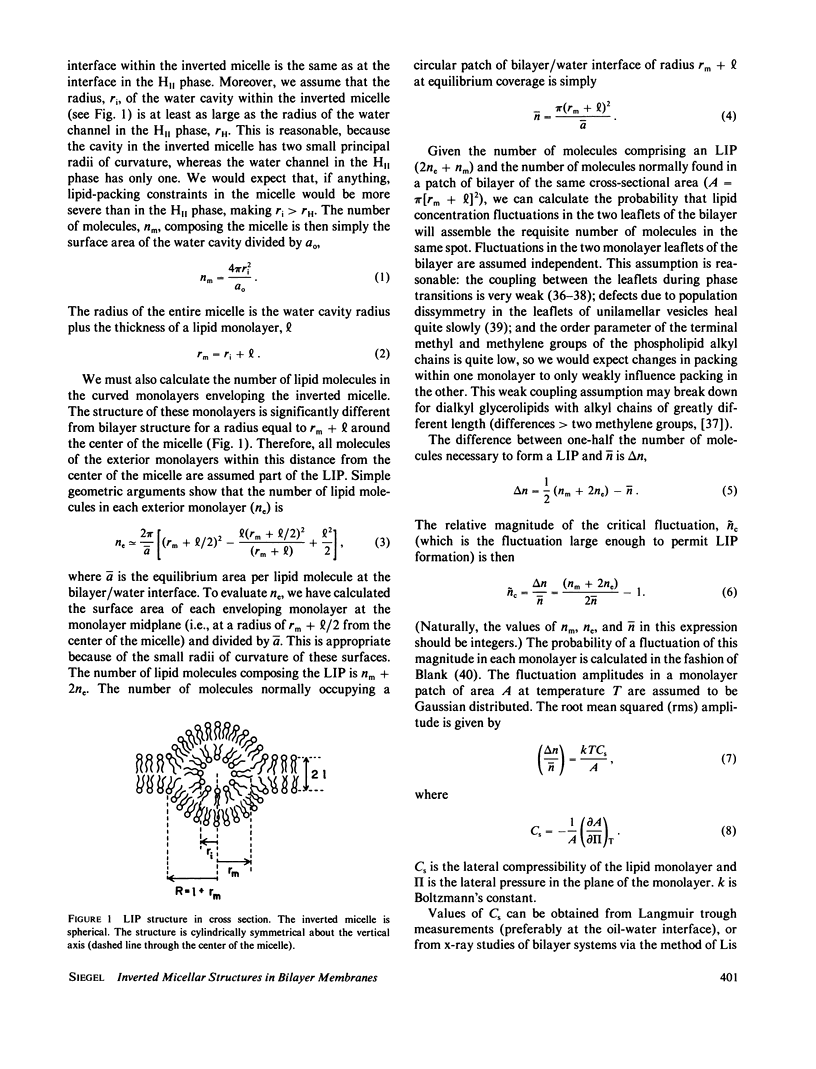
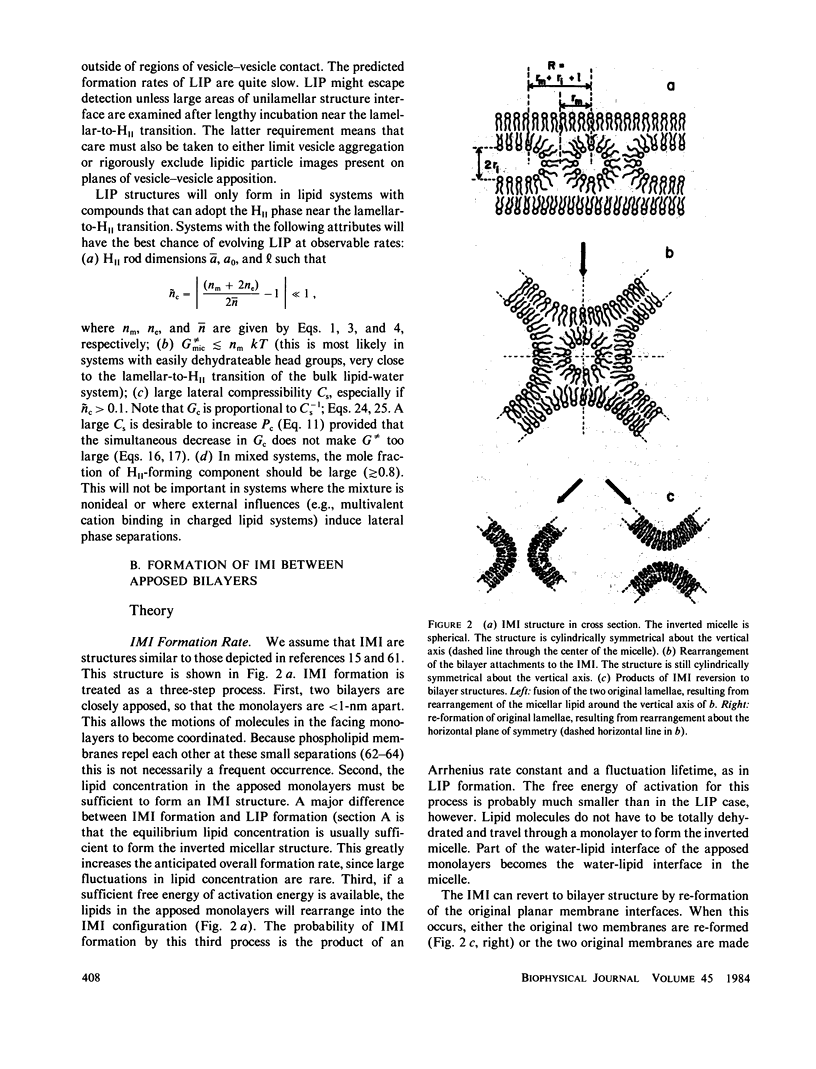
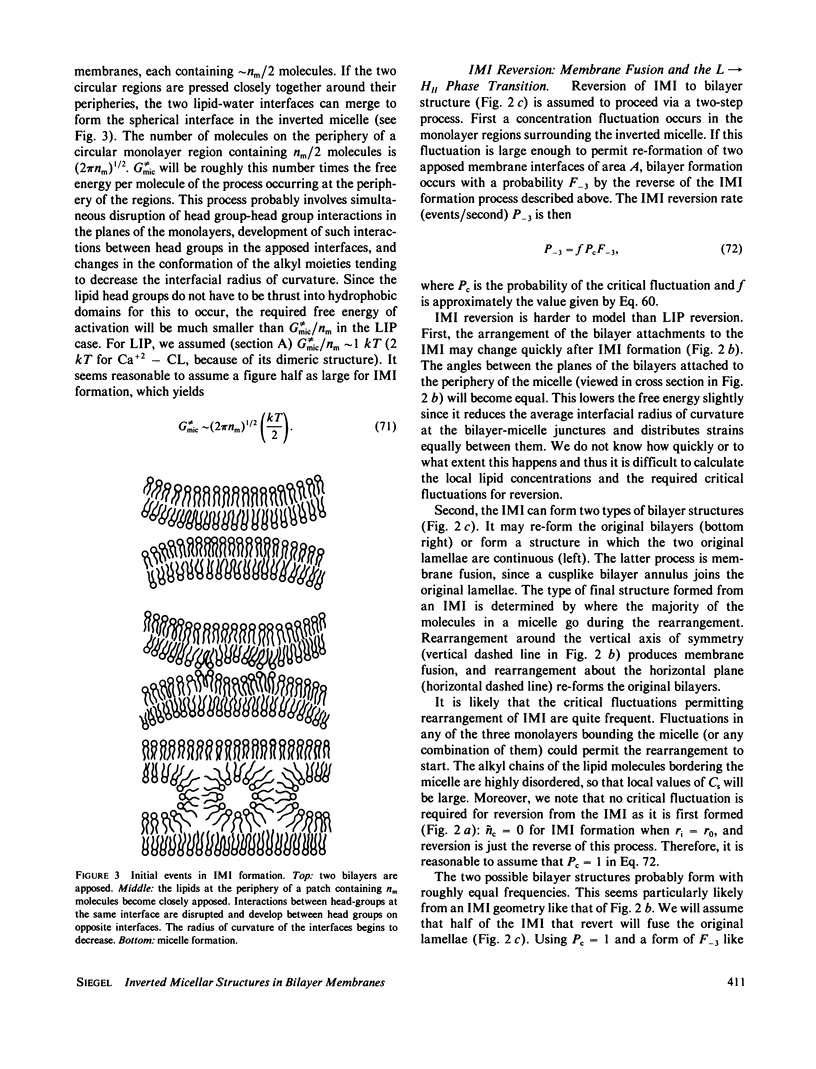
Two sorts of inverted micellar structures have previously been proposed to explain morphological and 31P-NMR observations of bilayer systems. These structures only form in systems with components that can adopt the inverse hexagonal (HII) phase. LIP (lipidic particles) are intrabilayer structures, whereas IMI (inverted micellar intermediates) are structures that form between apposed bilayers. Here, we calculate the formation rates and half-lives of these structures to determine which (or if either) of these proposed structures is a likely explanation of the data. Calculations for the egg phosphatidylethanolamine and the Ca+-cardiolipin systems show that IMI form orders of magnitude faster than LIP, which should form slowly, if at all. This result is probably true in general, and indicates that "lipidic particle" electron micrograph images probably represent interbilayer structures, as some have previously proposed. It is shown here that IMI are likely intermediates in the lamellar----HII phase transitions and in the process of membrane fusion in some systems. The calculated formation rates, half-lives, and vesicle-vesicle fusion rates are in agreement with this observation.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearer E. L., Düzgünes N., Friend D. S., Papahadjopoulos D. Fusion of phospholipid vesicles arrested by quick-freezing. The question of lipidic particles as intermediates in membrane fusion. Biochim Biophys Acta. 1982 Dec 8;693(1):93–98. doi: 10.1016/0005-2736(82)90474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette C. G., Segrest J. P., Ng T. C., Jones J. L. Minimal size phosphatidylcholine vesicles: effects of radius of curvature on head group packing and conformation. Biochemistry. 1982 Sep 14;21(19):4569–4575. doi: 10.1021/bi00262a009. [DOI] [PubMed] [Google Scholar]
- Burnell E. E., Cullis P. R., de Kruijff B. Effects of tumbling and lateral diffusion on phosphatidylcholine model membrane 31P-NMR lineshapes. Biochim Biophys Acta. 1980 Dec 2;603(1):63–69. doi: 10.1016/0005-2736(80)90391-0. [DOI] [PubMed] [Google Scholar]
- Cornell B. A., Fletcher G. C., Middlehurst J., Separovic F. The lower limit to the size of small sonicated phospholipid vesicles. Biochim Biophys Acta. 1982 Aug 25;690(1):15–19. doi: 10.1016/0005-2736(82)90233-4. [DOI] [PubMed] [Google Scholar]
- Cowley A. C., Fuller N. L., Rand R. P., Parsegian V. A. Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry. 1978 Jul 25;17(15):3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., De Kruijff B. Polymorphic phase behaviour of lipid mixtures as detected by 31P NMR. Evidence that cholesterol may destabilize bilayer structure in membrane systems containing phosphatidylethanolamine. Biochim Biophys Acta. 1978 Feb 21;507(2):207–218. doi: 10.1016/0005-2736(78)90417-0. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., De Kruyff B. 31P NMR studies of unsonicated aqueous dispersions of neutral and acidic phospholipids. Effects of phase transitions, p2H and divalent cations on the motion in the phosphate region of the polar headgroup. Biochim Biophys Acta. 1976 Jul 1;436(3):523–540. doi: 10.1016/0005-2736(76)90438-7. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J. Effects of fusogenic agent on membrane structure of erythrocyte ghosts and the mechanism of membrane fusion. Nature. 1978 Feb 16;271(5646):672–674. doi: 10.1038/271672a0. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B., Hope M. J., Nayar R., Schmid S. L. Phospholipids and membrane transport. Can J Biochem. 1980 Oct;58(10):1091–1100. doi: 10.1139/o80-147. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. The polymorphic phase behaviour of phosphatidylethanolamines of natural and synthetic origin. A 31P NMR study. Biochim Biophys Acta. 1978 Oct 19;513(1):31–42. doi: 10.1016/0005-2736(78)90109-8. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., van Dijck P. W., de Kruijff B., de Gier J. Effects of cholesterol on the properties of equimolar mixtures of synthetic phosphatidylethanolamine and phosphatidylcholine. A 31P NMR and differential scanning calorimetry study. Biochim Biophys Acta. 1978 Oct 19;513(1):21–30. doi: 10.1016/0005-2736(78)90108-6. [DOI] [PubMed] [Google Scholar]
- De Kruijff B., Verkleij A. J., Leunissen-Bijvelt J., Van Echteld C. J., Hille J., Rijnbout H. Further aspects of the Ca2+-dependent polymorphism of bovine heart cardiolipin. Biochim Biophys Acta. 1982 Dec 8;693(1):1–12. doi: 10.1016/0005-2736(82)90464-3. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Wilschut J., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion. Role of head-group composition in calcium- and magnesium-induced fusion of mixed phospholipid vesicles. Biochim Biophys Acta. 1981 Mar 20;642(1):182–195. doi: 10.1016/0005-2736(81)90148-6. [DOI] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Farren S. B., Cullis P. R. Polymorphism of phosphatidylglycerol-phosphatidylethanolamine model membrane systems: a 31p NMR study. Biochem Biophys Res Commun. 1980 Nov 17;97(1):182–191. doi: 10.1016/s0006-291x(80)80152-5. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Hardman P. D. Spin-label characterisation of the lamellar-to-hexagonal (HII) phase transition in egg phosphatidylethanolamine aqueous dispersions. Eur J Biochem. 1982 May;124(1):95–101. doi: 10.1111/j.1432-1033.1982.tb05910.x. [DOI] [PubMed] [Google Scholar]
- Harlos K., Eibl H. Hexagonal phases in phospholipids with saturated chains: phosphatidylethanolamines and phosphatidic acids. Biochemistry. 1981 May 12;20(10):2888–2892. doi: 10.1021/bi00513a027. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Martin O. C. Transbilayer redistribution of phosphatidylethanolamine during fusion of phospholipid vesicles. Dependence on fusion rate, lipid phase separation, and formation of nonbilayer structures. Biochemistry. 1982 Nov 23;21(24):6097–6103. doi: 10.1021/bi00267a011. [DOI] [PubMed] [Google Scholar]
- Hoekstra D. Role of lipid phase separations and membrane hydration in phospholipid vesicle fusion. Biochemistry. 1982 Jun 8;21(12):2833–2840. doi: 10.1021/bi00541a004. [DOI] [PubMed] [Google Scholar]
- Hope M. J., Cullis P. R. The role of nonbilayer lipid structures in the fusion of human erythrocytes induced by lipid fusogens. Biochim Biophys Acta. 1981 Jan 8;640(1):82–90. doi: 10.1016/0005-2736(81)90533-2. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Boni L. T. Lipidic particles and cubic phase lipids. Nature. 1982 Mar 11;296(5853):175–176. doi: 10.1038/296175a0. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P. 'Lipidic particles' are intermembrane attachment sites. Nature. 1981 Apr 2;290(5805):427–428. doi: 10.1038/290427a0. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P., Yeagle P. L., Albert A. D. Bilayer to non-bilayer transition in mixtures of phosphatidylethanolamine and phosphatidylcholine: implications for membrane properties. Arch Biochem Biophys. 1981 Apr 1;207(2):227–240. doi: 10.1016/0003-9861(81)90029-1. [DOI] [PubMed] [Google Scholar]
- Junger E., Reinauer H. Liquid crystalline phases of hydrated phosphatidylethanolamine. Biochim Biophys Acta. 1969 Jul 15;183(2):304–308. doi: 10.1016/0005-2736(69)90086-8. [DOI] [PubMed] [Google Scholar]
- Kolber M. A., Haynes D. H. Evidence for a role of phosphatidyl ethanolamine as a modulator of membrane-membrane contact. J Membr Biol. 1979 Jun 29;48(1):95–114. doi: 10.1007/BF01869258. [DOI] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawaczeck R., Kainosho M., Chan S. I. The formation and annealing of structural defects in lipid bilayer vesicles. Biochim Biophys Acta. 1976 Sep 7;443(3):313–330. doi: 10.1016/0005-2736(76)90032-8. [DOI] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Interactions between neutral phospholipid bilayer membranes. Biophys J. 1982 Mar;37(3):657–665. [PMC free article] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Measurement of the lateral compressibility of several phospholipid bilayers. Biophys J. 1982 Mar;37(3):667–672. [PMC free article] [PubMed] [Google Scholar]
- Mandersloot J. G., Gerritsen W. J., Leunissen-Bijvelt J., van Echteld C. J., Noordam P. C., de Gier J. Ca2+-induced changes in the barrier properties of cardiolipin/phosphatidylcholine bilayers. Biochim Biophys Acta. 1981 Jan 8;640(1):106–113. doi: 10.1016/0005-2736(81)90536-8. [DOI] [PubMed] [Google Scholar]
- Miller R. G. Do 'lipidic particles' represent intermembrane attachment sites? Nature. 1980 Sep 11;287(5778):166–167. doi: 10.1038/287166a0. [DOI] [PubMed] [Google Scholar]
- Nayar R., Schmid S. L., Hope M. J., Cullis P. R. Structural preferences of phosphatidylinositol and phosphatidylinositol-phosphatidylethanolamine model membranes. Influence of Ca2+ and Mg2+. Biochim Biophys Acta. 1982 May 21;688(1):169–176. doi: 10.1016/0005-2736(82)90592-2. [DOI] [PubMed] [Google Scholar]
- Nir S., Wilschut J., Bentz J. The rate of fusion of phospholipid vesicles and the role of bilayer curvature. Biochim Biophys Acta. 1982 May 21;688(1):275–278. doi: 10.1016/0005-2736(82)90604-6. [DOI] [PubMed] [Google Scholar]
- Nir S., Wilschut J., Bentz J. The rate of fusion of phospholipid vesicles and the role of bilayer curvature. Biochim Biophys Acta. 1982 May 21;688(1):275–278. doi: 10.1016/0005-2736(82)90604-6. [DOI] [PubMed] [Google Scholar]
- Noordam P. C., van Echteld C. J., de Kruijff B., Verkleij A. J., de Gier J. Barrier characteristics of membrane model systems containing unsaturated phosphatidylethanolamines. Chem Phys Lipids. 1980 Oct;27(3):221–232. doi: 10.1016/0009-3084(80)90037-7. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Newton C., Nir S., Jacobson K., Poste G., Lazo R. Studies on membrane fusion. III. The role of calcium-induced phase changes. Biochim Biophys Acta. 1977 Mar 17;465(3):579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- Portis A., Newton C., Pangborn W., Papahadjopoulos D. Studies on the mechanism of membrane fusion: evidence for an intermembrane Ca2+-phospholipid complex, synergism with Mg2+, and inhibition by spectrin. Biochemistry. 1979 Mar 6;18(5):780–790. doi: 10.1021/bi00572a007. [DOI] [PubMed] [Google Scholar]
- Rand R. P. Interacting phospholipid bilayers: measured forces and induced structural changes. Annu Rev Biophys Bioeng. 1981;10:277–314. doi: 10.1146/annurev.bb.10.060181.001425. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Reese T. S., Miller R. G. Phospholipid bilayer deformations associated with interbilayer contact and fusion. Nature. 1981 Sep 17;293(5829):237–238. doi: 10.1038/293237a0. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Sengupta S. Cardiolipin forms hexagonal structures with divalent cations. Biochim Biophys Acta. 1972 Feb 11;255(2):484–492. doi: 10.1016/0005-2736(72)90152-6. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAH D. O., SCHULMAN J. H. BINDING OF METAL IONS TO MONOLAYERS OF LECITHINS, PLASMALOGEN, CARDIOLIPIN, AND DICETYL PHOSPHATE. J Lipid Res. 1965 Jul;6:341–349. [PubMed] [Google Scholar]
- Schmidt C. F., Barenholz Y., Huang C., Thompson T. E. Monolayer coupling in sphingomyelin bilayer systems. Nature. 1978 Feb 23;271(5647):775–777. doi: 10.1038/271775a0. [DOI] [PubMed] [Google Scholar]
- Sen A., Brain A. P., Quinn P. J., Williams W. P. Formation of inverted lipid micelles in aqueous dispersions of mixed sn-3-galactosyldiacylglycerols induced by heat and ethylene glycol. Biochim Biophys Acta. 1982 Apr 7;686(2):215–224. doi: 10.1016/0005-2736(82)90115-8. [DOI] [PubMed] [Google Scholar]
- Sillerud L. O., Barnett R. E. Lack of transbilayer coupling in phase transitions of phosphatidylcholine vesicles. Biochemistry. 1982 Apr 13;21(8):1756–1760. doi: 10.1021/bi00537a009. [DOI] [PubMed] [Google Scholar]
- Sundler R., Düzgüneş N., Papahadjopoulos D. Control of membrane fusion by phospholipid head groups. II. The role of phosphatidylethanolamine in mixtures with phosphatidate and phosphatidylinositol. Biochim Biophys Acta. 1981 Dec 21;649(3):751–758. doi: 10.1016/0005-2736(81)90180-2. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Bally M. B., Farren S. B., Cullis P. R. Influence of cholesterol on the structural preferences of dioleoylphosphatidylethanolamine-dioleoylphosphatidylcholine systems: a phosphorus-31 and deuterium nuclear magnetic resonance study. Biochemistry. 1982 Sep 14;21(19):4596–4601. doi: 10.1021/bi00262a013. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Cullis P. R. The polymorphic phase behaviour of mixed phosphatidylserine-phosphatidylethanolamine model systems as detected by 31P-NMR. Biochim Biophys Acta. 1981 Feb 20;641(1):189–201. doi: 10.1016/0005-2736(81)90583-6. [DOI] [PubMed] [Google Scholar]
- Tinker D. O., Pinteric L. On the identification of lamellar and hexagonal phases in negatively stained phospholipid-water systems. Biochemistry. 1971 Mar 2;10(5):860–865. doi: 10.1021/bi00781a020. [DOI] [PubMed] [Google Scholar]
- Van Venetie R., Verkleij A. J. Analysis of the hexagonal II phase and its relations to lipidic particles and the lamellar phase. A freeze-fracture study. Biochim Biophys Acta. 1981 Jul 20;645(2):262–269. doi: 10.1016/0005-2736(81)90197-8. [DOI] [PubMed] [Google Scholar]
- Vasilenko I., De Kruijff B., Verkleij A. J. The synthesis and use of thionphospholipids in 31P-NRM studies of lipid polymorphism. Biochim Biophys Acta. 1982 Feb 23;685(2):144–152. doi: 10.1016/0005-2736(82)90091-8. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., De Maagd R., Leunissen-Bijvelt J., De Kruijff B. Divalent cations and chlorpromazine can induce non-bilayer structures in phosphatidic acid-containing model membranes. Biochim Biophys Acta. 1982 Jan 22;684(2):255–262. doi: 10.1016/0005-2736(82)90014-1. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Mombers C., Gerritsen W. J., Leunissen-Bijvelt L., Cullis P. R. Fusion of phospholipid vesicles in association with the appearance of lipidic particles as visualized by freeze fracturing. Biochim Biophys Acta. 1979 Aug 7;555(2):358–361. doi: 10.1016/0005-2736(79)90175-5. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Momvers C., Leunissen-Bijvelt J., Ververgaert P. H. Lipidic intramembranous particles. Nature. 1979 May 10;279(5709):162–163. doi: 10.1038/279162a0. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., van Echteld C. J., Gerritsen W. J., Cullis P. R., de Kruijff B. The lipidic particle as an intermediate structure in membrane fusion processes and bilayer to hexagonal HII transitions. Biochim Biophys Acta. 1980 Aug 14;600(3):620–624. doi: 10.1016/0005-2736(80)90465-4. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry. 1980 Dec 23;19(26):6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Papahadjopoulos D. Calcium/magnesium specificity in membrane fusion: kinetics of aggregation and fusion of phosphatidylserine vesicles and the role of bilayer curvature. Biochemistry. 1981 May 26;20(11):3126–3133. doi: 10.1021/bi00514a022. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Holsappel M., Jansen R. Ca2+-induced fusion of cardiolipin/phosphatidylcholine vesicles monitored by mixing of aqueous contents. Biochim Biophys Acta. 1982 Sep 9;690(2):297–301. doi: 10.1016/0005-2736(82)90334-0. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Papahadjopoulos D. Ca2+-induced fusion of phospholipid vesicles monitored by mixing of aqueous contents. Nature. 1979 Oct 25;281(5733):690–692. doi: 10.1038/281690a0. [DOI] [PubMed] [Google Scholar]
- Wolfe J., Steponkus P. L. The stress-strain relation of the plasma membrane of isolated plant protoplasts. Biochim Biophys Acta. 1981 May 20;643(3):663–668. doi: 10.1016/0005-2736(81)90363-1. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Verkley A. J., van Echteld C. J., Gerritsen W. J., Mombers C., Noordam P. C., de Gier J. The occurrence of lipidic particles in lipid bilayers as seen by 31P NMR and freeze-fracture electron-microscopy. Biochim Biophys Acta. 1979 Aug 7;555(2):200–209. doi: 10.1016/0005-2736(79)90160-3. [DOI] [PubMed] [Google Scholar]


