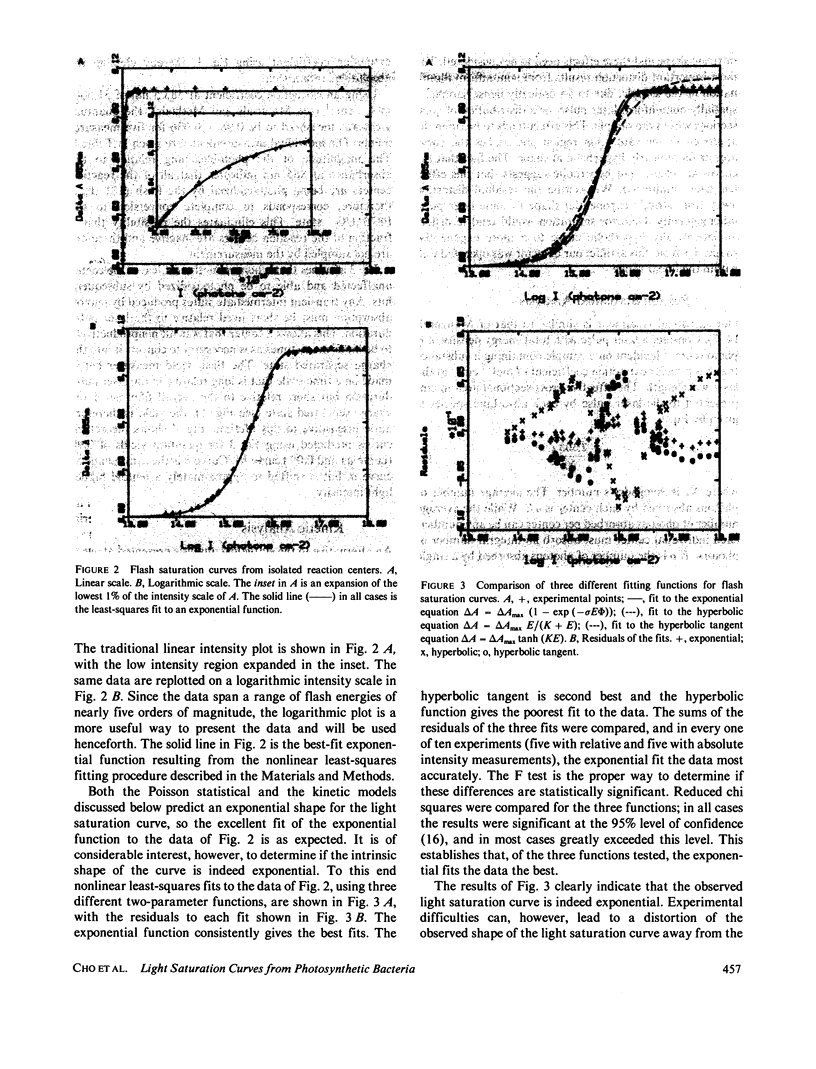
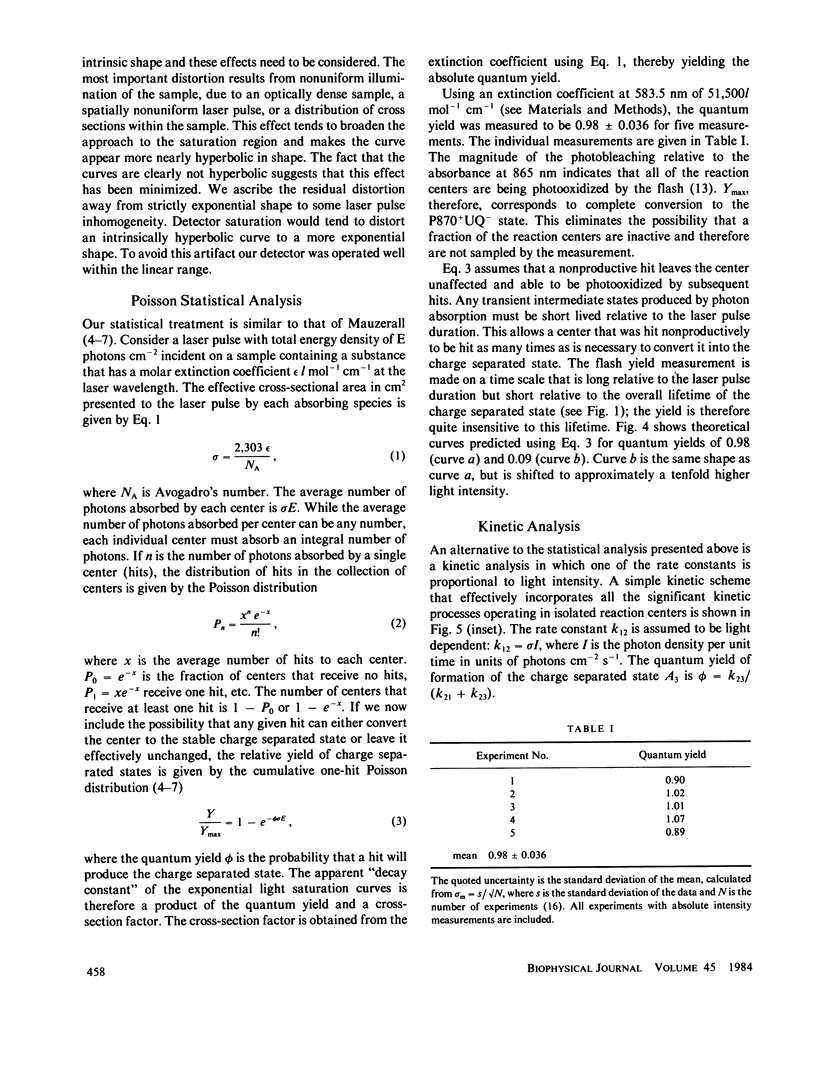
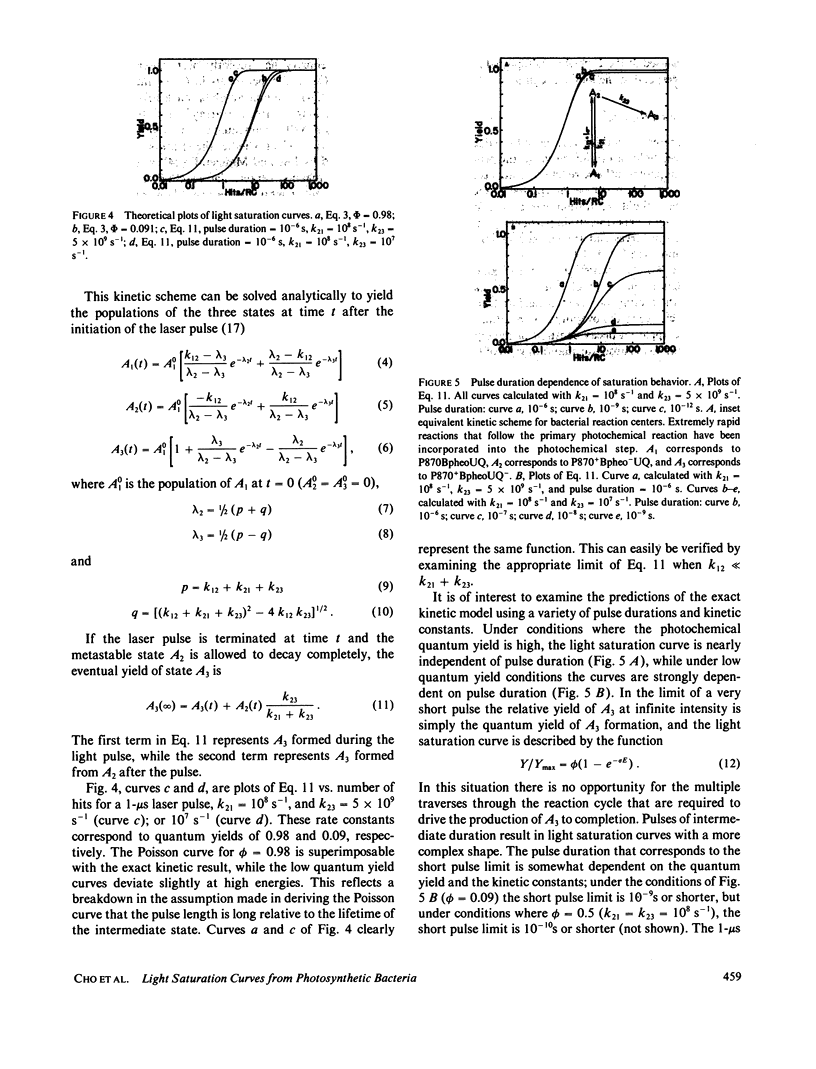
Abstract
Reaction centers isolated from the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26 mutant were irradiated with laser pulses of variable energy and the amount of photooxidation of the primary electron donor bacteriochlorophyll was measured. The resultant light saturation curve fits an exponential function and not a hyperbolic or hyperbolic tangent function. Analysis using either a Poisson statistical model or a simple kinetic model predicts an exponential light saturation curve in the limit where the light pulse is long relative to any transient intermediate states. The absolute quantum yield of photochemistry was found to be 0.98, utilizing the entire light saturation curve. Distortions from the simple exponential light saturation behavior are predicted when very short laser pulses are used.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship R. E., Parson W. W. The photochemical electron transfer reactions of photosynthetic bacteria and plants. Annu Rev Biochem. 1978;47:635–653. doi: 10.1146/annurev.bi.47.070178.003223. [DOI] [PubMed] [Google Scholar]
- Breton J., Geacintov N. E. Picosecond fluorescence kinetics and fast energy transfer processes in photosynthetic membranes. Biochim Biophys Acta. 1980 Dec 22;594(1):1–32. doi: 10.1016/0304-4173(80)90011-7. [DOI] [PubMed] [Google Scholar]
- Chalker B. E. Modelling light saturation curves for photosynthesis: an exponential function. J Theor Biol. 1980 May 21;84(2):205–213. doi: 10.1016/s0022-5193(80)80004-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Dutton P. L., Netzel T. L., Leigh J. S., Rentzepis P. M. Picosecond kinetics of events leading to reaction center bacteriochlorophyll oxidation. Science. 1975 Jun 27;188(4195):1301–1304. doi: 10.1126/science.188.4195.1301. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Sekura D. L. Primary photochemistry and electron transport in Rhodospirillum rubrum. Biochemistry. 1968 Jul;7(7):2642–2649. doi: 10.1021/bi00847a029. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Rockley M. G., Windsor M. W., Cogdell R. J., Parson W. W. Picosecond detection of an intermediate in the photochemical reaction of bacterial photosynthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2251–2255. doi: 10.1073/pnas.72.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Parson W. W., Mauzerall D. C., Clayton R. K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jun 28;305(3):597–609. doi: 10.1016/0005-2728(73)90079-0. [DOI] [PubMed] [Google Scholar]


