Abstract
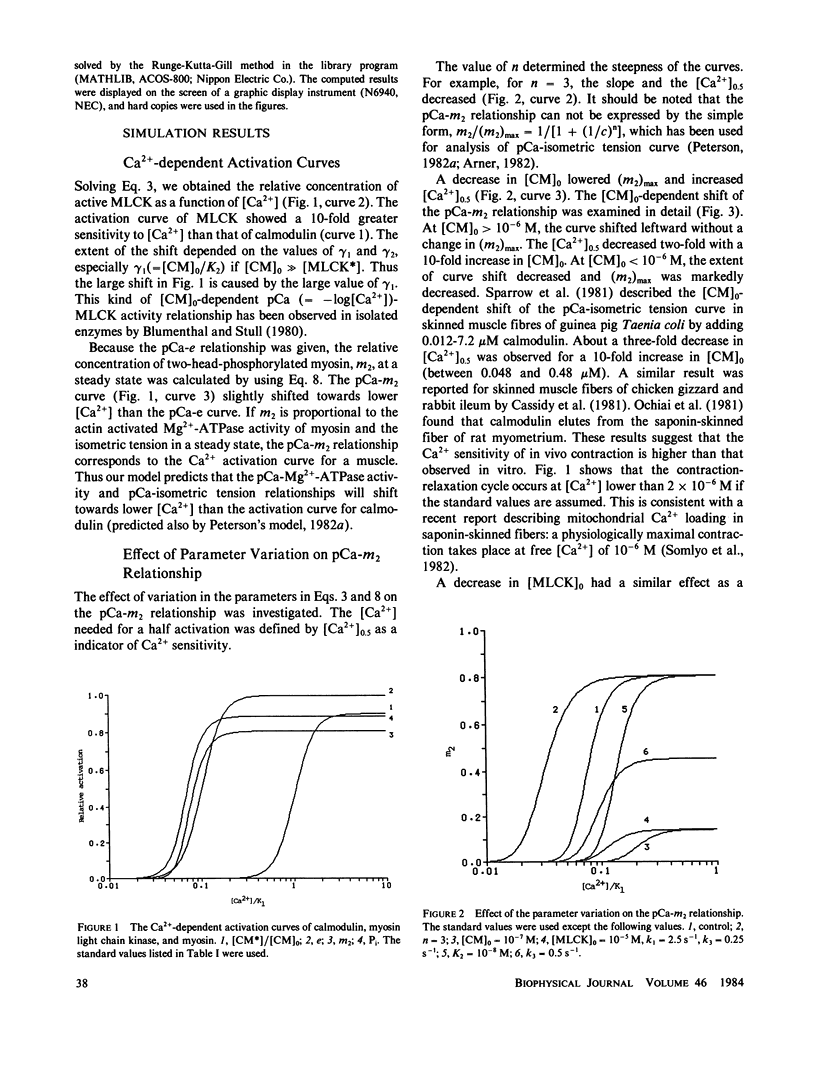
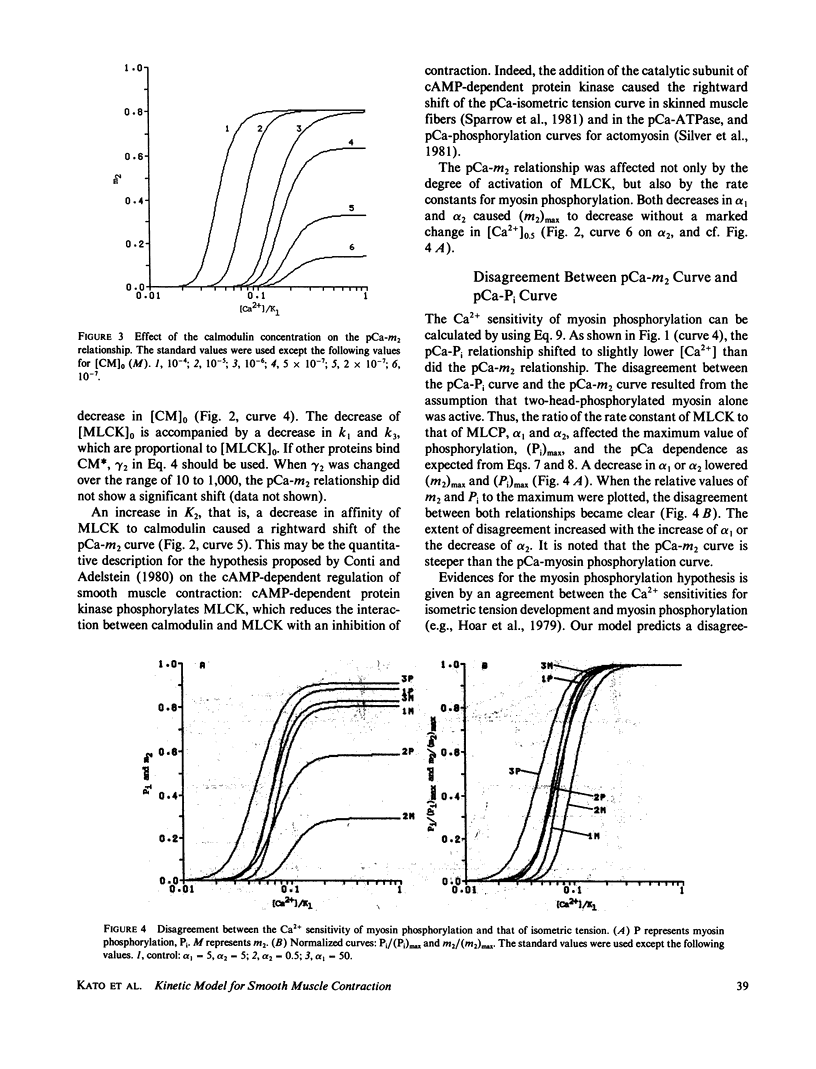
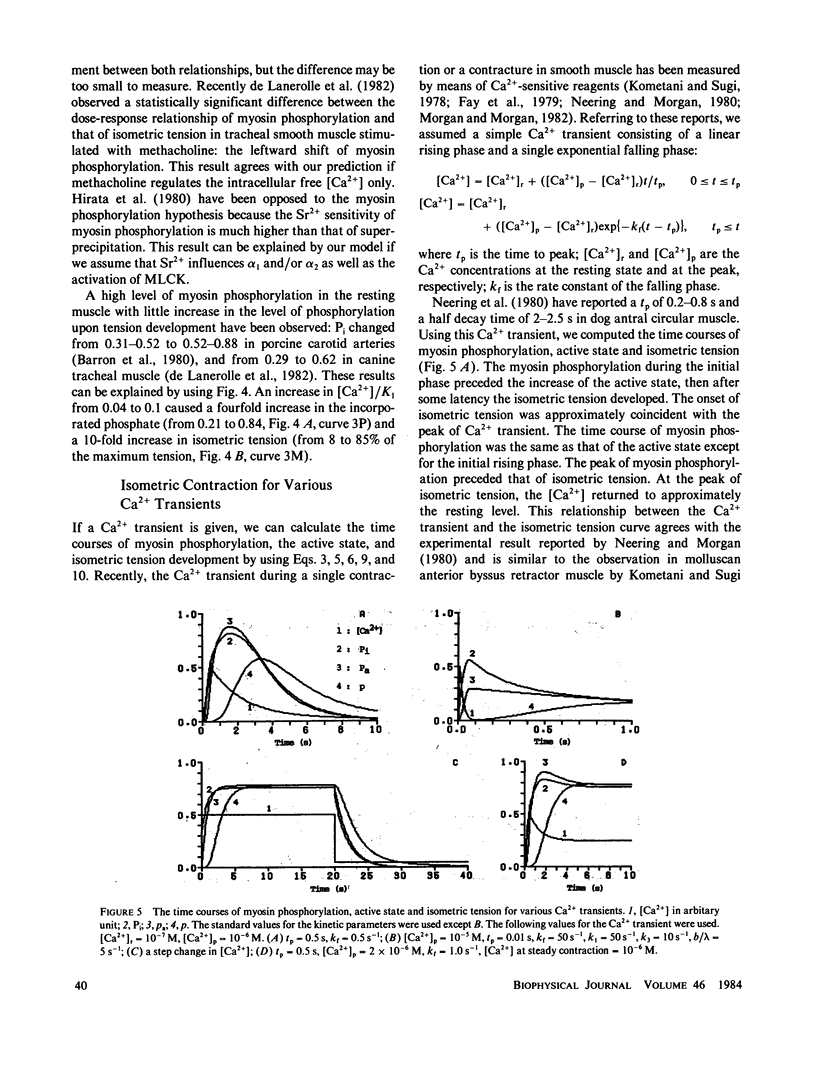
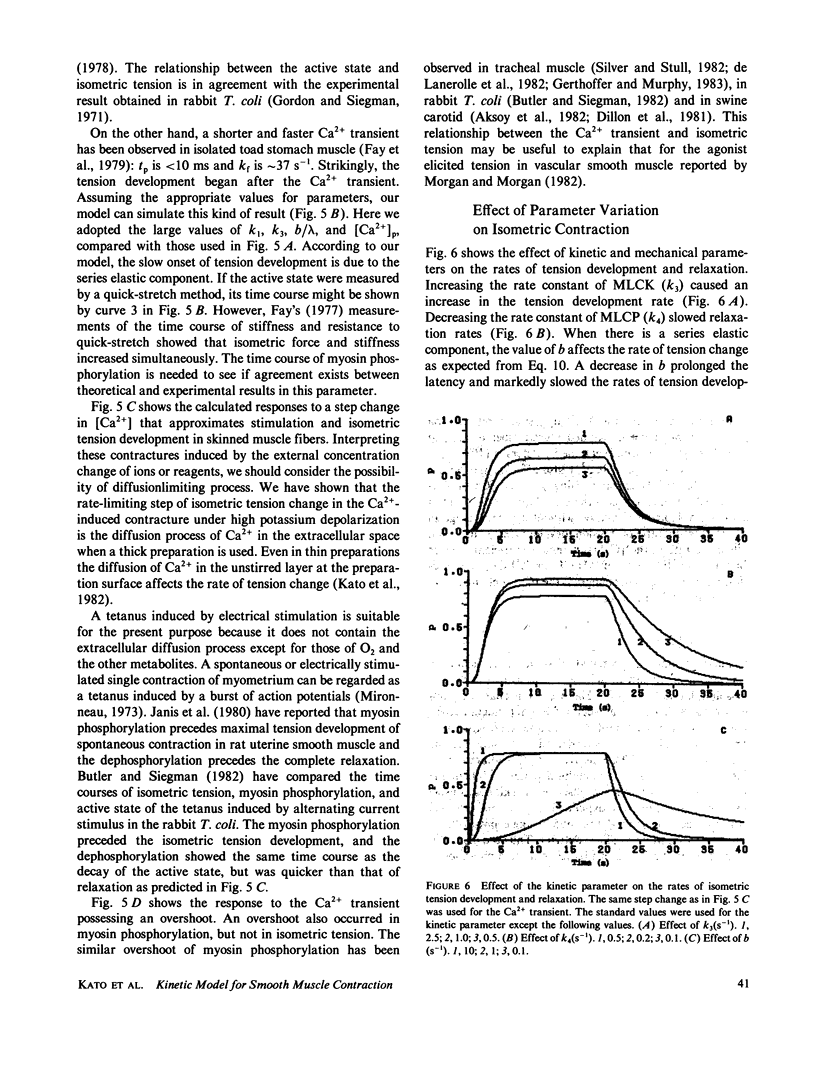
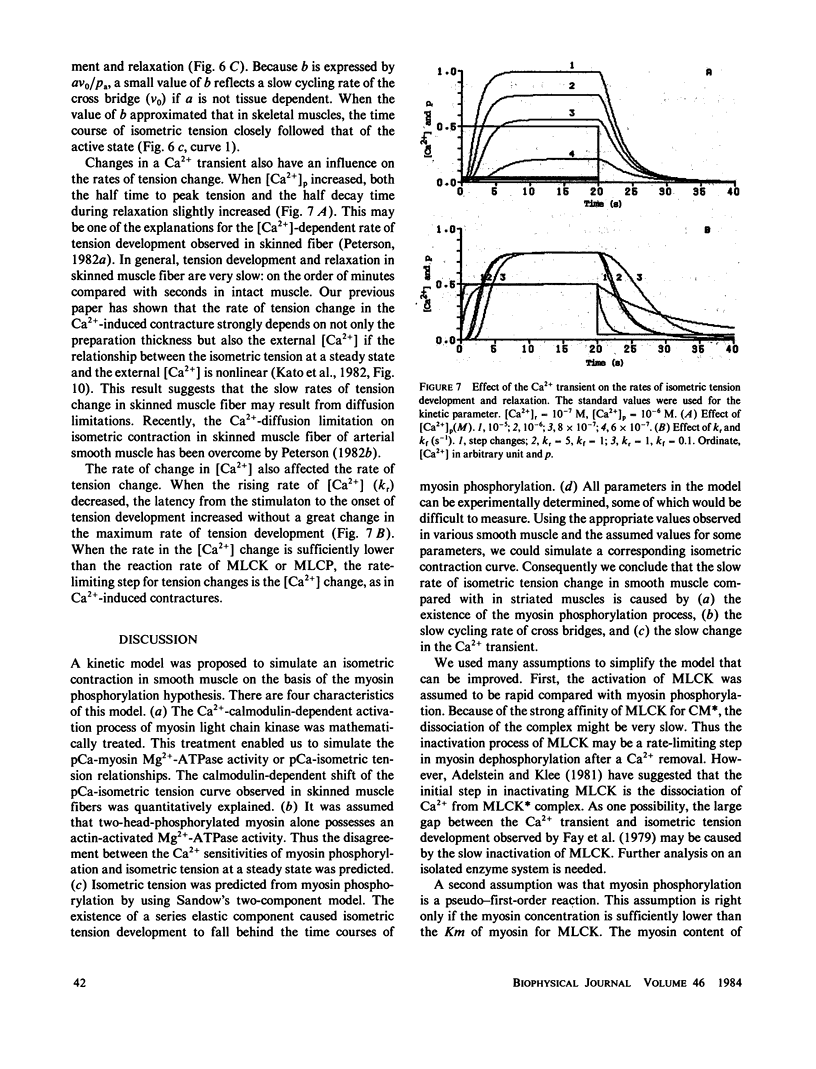
A kinetic model was proposed to simulate an isometric contraction curve in smooth muscle on the basis of the myosin phosphorylation hypothesis. The Ca2+-calmodulin-dependent activation of myosin light-chain kinase and the phosphorylation-dephosphorylation reaction of myosin were mathematically treated. Solving the kinetic equations at a steady state, we could calculate the relationship between the Ca2+ concentration and the myosin phosphorylation. Assuming that two-head-phosphorylated myosin has an actin-activated Mg2+-ATPase activity and that this state corresponds to an active state, we computed the time courses of the myosin phosphorylation and the active state for various Ca2+ transients. The time course of the active state was converted into that of isometric tension by use of Sandow's model composed of a contractile element and a series elastic component. The model could simulate not only the isometric contraction curves for any given Ca2+ transient but also the following experimental results: the calmodulin-dependent shift of the Ca2+ sensitivity of isometric tension observed in skinned muscle fibers, the disagreement between the Ca2+ sensitivity of myosin phosphorylation and that of isometric tension at a steady state, and the disagreement between the time course of myosin phosphorylation and that of isometric tension development.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Klee C. B. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981 Jul 25;256(14):7501–7509. [PubMed] [Google Scholar]
- Aksoy M. O., Murphy R. A., Kamm K. E. Role of Ca2+ and myosin light chain phosphorylation in regulation of smooth muscle. Am J Physiol. 1982 Jan;242(1):C109–C116. doi: 10.1152/ajpcell.1982.242.1.C109. [DOI] [PubMed] [Google Scholar]
- Barron J. T., Bárány M., Bárány K., Storti R. V. Reversible phosphorylation and dephosphorylation of the 20,000-dalton light chain of myosin during the contraction-relaxation-contraction cycle of arterial smooth muscle. J Biol Chem. 1980 Jul 10;255(13):6238–6244. [PubMed] [Google Scholar]
- Blumenthal D. K., Stull J. T. Activation of skeletal muscle myosin light chain kinase by calcium(2+) and calmodulin. Biochemistry. 1980 Nov 25;19(24):5608–5614. doi: 10.1021/bi00565a023. [DOI] [PubMed] [Google Scholar]
- Butler T. M., Siegman M. J. Chemical Energetics of contraction in mammalian smooth muscle. Fed Proc. 1982 Feb;41(2):204–208. [PubMed] [Google Scholar]
- Cassidy P. S., Kerrick W. G., Hoar P. E., Malencik D. A. Exogenous calmodulin increases Ca2+ sensitivity of isometric tension activation and myosin phosphorylation in skinned smooth muscle. Pflugers Arch. 1981 Dec;392(2):115–120. doi: 10.1007/BF00581258. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Hoar P. E., Kerrick W. G. Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATP gamma S. J Biol Chem. 1979 Nov 10;254(21):11148–11153. [PubMed] [Google Scholar]
- Cohen D. M., Murphy R. A. Differences in cellular contractile protein contents among porcine smooth muscles: evidence for variation in the contractile system. J Gen Physiol. 1978 Sep;72(3):369–380. doi: 10.1085/jgp.72.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. Phosphorylation by cyclic adenosine 3':5'-monophosphate-dependent protein kinase regulates myosin light chain kinase. Fed Proc. 1980 Apr;39(5):1569–1573. [PubMed] [Google Scholar]
- Crouch T. H., Klee C. B. Positive cooperative binding of calcium to bovine brain calmodulin. Biochemistry. 1980 Aug 5;19(16):3692–3698. doi: 10.1021/bi00557a009. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Hinkins S., Walsh M. P., Hartshorne D. J. The binding of smooth muscle myosin light chain kinase to actin. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1524–1531. doi: 10.1016/s0006-291x(82)80172-1. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Sherry J. M., Aromatorio D. K., Hartshorne D. J. Modulator protein as a component of the myosin light chain kinase from chicken gizzard. Biochemistry. 1978 Jan 24;17(2):253–258. doi: 10.1021/bi00595a010. [DOI] [PubMed] [Google Scholar]
- Dillon P. F., Aksoy M. O., Driska S. P., Murphy R. A. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981 Jan 30;211(4481):495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- Dillon P. F., Murphy R. A. Tonic force maintenance with reduced shortening velocity in arterial smooth muscle. Am J Physiol. 1982 Jan;242(1):C102–C108. doi: 10.1152/ajpcell.1982.242.1.C102. [DOI] [PubMed] [Google Scholar]
- Driska S. P., Aksoy M. O., Murphy R. A. Myosin light chain phosphorylation associated with contraction in arterial smooth muscle. Am J Physiol. 1981 May;240(5):C222–C233. doi: 10.1152/ajpcell.1981.240.5.C222. [DOI] [PubMed] [Google Scholar]
- Fay F. S. Isometric contractile properties of single isolated smooth muscle cells. Nature. 1977 Feb 10;265(5594):553–556. doi: 10.1038/265553a0. [DOI] [PubMed] [Google Scholar]
- Fay F. S., Shlevin H. H., Granger W. C., Jr, Taylor S. R. Aequorin luminescence during activation of single isolated smooth muscle cells. Nature. 1979 Aug 9;280(5722):506–508. doi: 10.1038/280506a0. [DOI] [PubMed] [Google Scholar]
- Frearson N., Focant B. W., Perry S. V. Phosphorylation of a light chain component of myosin from smooth muscle. FEBS Lett. 1976 Mar 15;63(1):27–32. doi: 10.1016/0014-5793(76)80187-1. [DOI] [PubMed] [Google Scholar]
- Gerthoffer W. T., Murphy R. A. Myosin phosphorylation and regulation of cross-bridge cycle in tracheal smooth muscle. Am J Physiol. 1983 Mar;244(3):C182–C187. doi: 10.1152/ajpcell.1983.244.3.C182. [DOI] [PubMed] [Google Scholar]
- Gordon A. R., Siegman M. J. Mechanical properties of smooth muscle. II. Active state. Am J Physiol. 1971 Nov;221(5):1250–1254. doi: 10.1152/ajplegacy.1971.221.5.1250. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V. The heat of activation and the heat of shortening in a muscle twitch. Proc R Soc Lond B Biol Sci. 1949 Jun 23;136(883):195–211. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Siemankowski R. F. Regulation of smooth muscle actomyosin. Annu Rev Physiol. 1981;43:519–530. doi: 10.1146/annurev.ph.43.030181.002511. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Asano M., Iwadare S., Matsumoto I., Totsuka T., Aoki N. A novel vascular relaxing agent, N-(6--aminohexyl)-5-chloro-1-naphthalensulfonamide which affects vascular smooth muscle actomyosin. J Pharmacol Exp Ther. 1978 Oct;207(1):8–15. [PubMed] [Google Scholar]
- Hirata M., Mikawa T., Nonomura Y., Ebashi S. Ca2+ regulation in vascular smooth muscle. II. Ca2+ binding of aorta leiotonin. J Biochem. 1980 Feb;87(2):369–378. doi: 10.1093/oxfordjournals.jbchem.a132757. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Kerrick W. G., Cassidy P. S. Chicken gizzard: relation between calcium-activated phosphorylation and contraction. Science. 1979 May 4;204(4392):503–506. doi: 10.1126/science.432654. [DOI] [PubMed] [Google Scholar]
- Janis R. A., Moats-Staats B. M., Gualtieri R. T. Protein phosphorylation during spontaneous contraction of smooth muscle. Biochem Biophys Res Commun. 1980 Sep 16;96(1):265–270. doi: 10.1016/0006-291x(80)91209-7. [DOI] [PubMed] [Google Scholar]
- Kato S., Ogasawara T., Osa T. Calcium diffusion in uterine smooth muscle sheets. J Gen Physiol. 1982 Aug;80(2):257–277. doi: 10.1085/jgp.80.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Hoar P. E., Cassidy P. S. Calcium-activated tension: the role of myosin light chain phosphorylation. Fed Proc. 1980 Apr;39(5):1558–1563. [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Marston S. B. The regulation of smooth muscle contractile proteins. Prog Biophys Mol Biol. 1983;41(1):1–41. doi: 10.1016/0079-6107(83)90024-x. [DOI] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Vascular smooth muscle: the first recorded Ca2+ transients. Pflugers Arch. 1982 Oct;395(1):75–77. doi: 10.1007/BF00584972. [DOI] [PubMed] [Google Scholar]
- Murphy R. A. Contractile system function in mammalian smooth muscle. Blood Vessels. 1976;13(1-2):1–23. doi: 10.1159/000158076. [DOI] [PubMed] [Google Scholar]
- Neering I. R., Morgan K. G. Use of aequorin to study excitation--contraction coupling in mammalian smooth muscle. Nature. 1980 Dec 11;288(5791):585–587. doi: 10.1038/288585a0. [DOI] [PubMed] [Google Scholar]
- Onishi H., Iijima S., Anzai H., Watanabe S. Possible role of myosin light-chain phosphatase in the relaxation of chicken gizzard muscle. J Biochem. 1979 Nov;86(5):1283–1290. doi: 10.1093/oxfordjournals.jbchem.a132644. [DOI] [PubMed] [Google Scholar]
- Pato M. D., Adelstein R. S. Dephosphorylation of the 20,000-dalton light chain of myosin by two different phosphatases from smooth muscle. J Biol Chem. 1980 Jul 25;255(14):6535–6538. [PubMed] [Google Scholar]
- Persechini A., Hartshorne D. J. Phosphorylation of smooth muscle myosin: evidence for cooperativity between the myosin heads. Science. 1981 Sep 18;213(4514):1383–1385. doi: 10.1126/science.6455737. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., 3rd Rate-limiting steps in the tension development of freeze-glycerinated vascular smooth muscle. J Gen Physiol. 1982 Mar;79(3):437–452. doi: 10.1085/jgp.79.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W., 3rd Simple model of smooth muscle myosin phosphorylation and dephosphorylation as rate-limiting mechanism. Biophys J. 1982 Feb;37(2):453–459. doi: 10.1016/S0006-3495(82)84691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDOW A. A theory of active state mechanisms in isometric muscular contraction. Science. 1958 Apr 4;127(3301):760–762. doi: 10.1126/science.127.3301.760. [DOI] [PubMed] [Google Scholar]
- Siegman M. J., Butler T. M., Mooers S. U., Davies R. E. Calcium-dependent resistance to stretch and stress relaxation in resting smooth muscles. Am J Physiol. 1976 Nov;231(5 Pt 1):1501–1508. doi: 10.1152/ajplegacy.1976.231.5.1501. [DOI] [PubMed] [Google Scholar]
- Silver P. J., Holroyde M. J., Solaro R. J., Disalvo J. Ca2+, calmodulin and cyclic AMP-dependent modulation of actin-myosin interactions in aorta. Biochim Biophys Acta. 1981 Apr 17;674(1):65–70. doi: 10.1016/0304-4165(81)90347-0. [DOI] [PubMed] [Google Scholar]
- Silver P. J., Stull J. T. Regulation of myosin light chain and phosphorylase phosphorylation in tracheal smooth muscle. J Biol Chem. 1982 Jun 10;257(11):6145–6150. [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H., Endo M. Calcium and monovalent ions in smooth muscle. Fed Proc. 1982 Oct;41(12):2883–2890. [PubMed] [Google Scholar]
- Sparrow M. P., Mrwa U., Hofmann F., Rüegg J. C. Calmodulin is essential for smooth muscle contraction. FEBS Lett. 1981 Mar 23;125(2):141–145. doi: 10.1016/0014-5793(81)80704-1. [DOI] [PubMed] [Google Scholar]
- Stephens N. L., Kromer U. Series elastic component of tracheal smooth muscle. Am J Physiol. 1971 Jun;220(6):1890–1895. doi: 10.1152/ajplegacy.1971.220.6.1890. [DOI] [PubMed] [Google Scholar]
- Taylor C. P. Isometric muscle contraction and the active state: an analog computer study. Biophys J. 1969 Jun;9(6):759–780. doi: 10.1016/s0006-3495(69)86416-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M. P., Bridenbaugh R., Hartshorne D. J., Kerrick W. G. Phosphorylation-dependent activated tension in skinned gizzard muscle fibers in the absence of Ca2+. J Biol Chem. 1982 Jun 10;257(11):5987–5990. [PubMed] [Google Scholar]
- Walsh M. P., Dabrowska R., Hinkins S., Hartshorne D. J. Calcium-independent myosin light chain kinase of smooth muscle. Preparation by limited chymotryptic digestion of the calcium ion dependent enzyme, purification, and characterization. Biochemistry. 1982 Apr 13;21(8):1919–1925. doi: 10.1021/bi00537a034. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Yagi K. Purification of modulator-deficient myosin light-chain kinase by modulator protein-Sepharose affinity chromatography. J Biochem. 1978 Nov;84(5):1259–1265. doi: 10.1093/oxfordjournals.jbchem.a132244. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P., Condit J. R., Jr, Tanenbaum M., Adelstein R. S. Myosin phosphorylation, agonist concentration and contraction of tracheal smooth muscle. Nature. 1982 Aug 26;298(5877):871–872. doi: 10.1038/298871a0. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P., Stull J. T. Myosin phosphorylation during contraction and relaxation of tracheal smooth muscle. J Biol Chem. 1980 Oct 25;255(20):9993–10000. [PubMed] [Google Scholar]


