Right ventricular function is an important prognostic factor for pulmonary embolism.1 Massive pulmonary embolism may lead to right ventricular failure, reduced left ventricular output, and even death.2 Cardiac troponins are routinely applied markers of minor and major myocardial damage in patients with acute coronary syndromes. In small case series, troponin concentrations were raised in patients with massive pulmonary embolism.3,4 The role of troponin as a prognostic factor is, however, unclear. We assessed the association between serum concentrations of cardiac troponin T and severity of pulmonary embolism as well as the role of troponin T as a predictor of mortality.
Participants, methods, and results
We assessed 136 consecutive patients who were admitted to the emergency department of a tertiary care university hospital between December 1999 and November 2001 with pulmonary embolism, confirmed by computed tomography or scintigraphy. Two patients with terminal illness and seven patients admitted after cardiac arrest out of hospital were excluded. In 106 patients troponin concentrations were determined in the first 12 hours after admission (Elecsys 2010; Roche, Mannheim, Germany). The severity of the event was classified according to the grading system by Grosser (see table A on bmj.com).5 Right ventricular strain in the electrocardiogram was defined as right bundle branch block, T wave inversion in precordial leads, or S1Q3T3 pattern. We used Spearman's correlation and the Wilcoxon rank sum test and constructed a receiver operating characteristic curve based on sensitivities and specificities, using various troponin values as cut offs to determine mortality in hospital. We used routine data; studies using such data are not routinely reviewed by the local ethical review board.
The median age of patients was 60 (interquartile range 43-72) years; 74 (58%) were female. Six had fulminant pulmonary embolism, in 37 it was massive, in 62 it was submassive, and in one it was minor. With increasing severity of pulmonary embolism troponin concentrations also increased (r=0.56, P<0.001). The median troponin concentration in patients with signs of right ventricular strain in the electrocardiogram was 0.03 ng/ml (interquartile range <0.01 to 0.06) and in patients without these signs <0.01 ng/ml (<0.01 to <0.01, P<0.001). Ninety three patients underwent echocardiography, of whom 63 (68%) had signs of right ventricular strain1; the median troponin concentration in patients with signs of right ventricular strain was 0.03 ng/ml compared with <0.01 ng/ml (P<0.001) in patients without right ventricular strain.
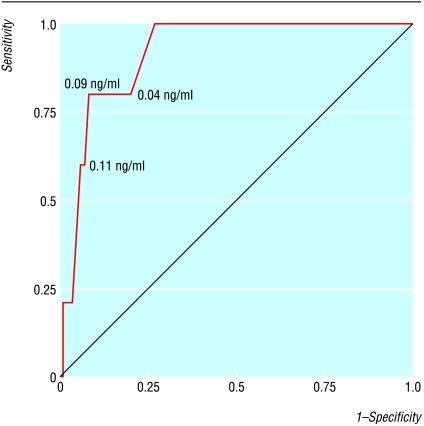
Five of 106 patients with troponin measurements died in hospital (5%); troponin concentrations were higher in patients who died than in survivors (0.18 ng/ml (0.09 to 0.18) v <0.01 ng/ml (<0.01 to 0.03), P<0.001). A cut-off value for troponin of 0.09 ng/ml was a suitable predictor for death in hospital (figure). The area under the curve was 0.92 (95% confidence interval 0.82 to 1.0), and the cut-off value had a sensitivity of 0.80 (0.49 to 1.0) and a specificity of 0.92 (0.87 to 0.97). The negative predictive value was 0.99 (0.93 to 1.00) and the positive predictive value 0.34 (0.10 to 0.59).
Comment
Raised concentrations of troponin T are associated with a higher in-hospital mortality in patients with pulmonary embolism. The major limitation of this study is that we do not know in how many patients pulmonary embolism remained undetected. We assume that missed cases had only minor symptoms and probably a good prognosis. Another limitation is that in 21 of the 127 eligible patients (17%) troponin was not measured. Patients with missing troponin values were younger (median 47 years v 60 years, P=0.014). The proportion of missing troponin values was similar in survivors and non-survivors (17%). Overall, we believe that this selection bias does not invalidate our conclusion. Whether troponin measurement can be used as a tool for clinical decision making—for example, deciding whether to give thrombolytic treatment—needs confirmation in larger prospective studies.
Supplementary Material
Figure.
Receiver operating characteristic curve of cardiac troponin T and mortality. The values at the curve indicate the respective concentrations of cardiac troponin T
Footnotes
Funding: None.
Competing interests: MM works part time as an editor with the BMJ but had nothing to do with the peer review of this paper.
An extra table appears on bmj.com
References
- 1.Kasper W, Konstantinides S, Geibel A, Tiede N, Krause T, Just H. Prognostic significance of right ventricular afterload stress detected by echocardiography in patients with clinically suspected pulmonary embolism. Heart. 1997;77:346–349. doi: 10.1136/hrt.77.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lualdi JC, Goldhaber SZ. Right ventricular dysfunction after acute pulmonary embolism: pathophysiologic factors, detection, and therapeutic implications. Am Heart J. 1995;130:1276–1282. doi: 10.1016/0002-8703(95)90155-8. [DOI] [PubMed] [Google Scholar]
- 3.Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36:1632–1636. doi: 10.1016/s0735-1097(00)00905-0. [DOI] [PubMed] [Google Scholar]
- 4.Giannitsis E, Muller-Bardorff M, Kurowski V, Weidtmann B, Wiegand U, Kampmann M, et al. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation. 2000;102:211–217. doi: 10.1161/01.cir.102.2.211. [DOI] [PubMed] [Google Scholar]
- 5.Grosser KD. Lungenembolie. Erkennung und differentialtherapeutische Probleme [Pulmonary embolism—problems in identifying and treating the condition] Internist. 1980;21:273–282. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.