Abstract
Liver imaging in patients with a history of known or suspected malignancy is important because the liver is a common site of metastatic spread, especially tumours from the colon, lung, pancreas and stomach, and in patients with chronic liver disease who are at risk for developing hepatocellular carcinoma. Since benign liver lesions are common, liver-imaging strategies should incorporate liver lesion detection and characterisation. Survey examination in patients with a known extra-hepatic malignancy to exclude the presence of hepatic and extra-hepatic involvement is normally undertaken with a contrast-enhanced computed tomography examination. When patients with hepatic metastases are being considered for metastasesectomy, they undergo a staging examination with contrast-enhanced magnetic resonance imaging (MRI) using tissue-specific contrast agents. Patients with chronic liver disease who are at risk for hepatocellular carcinoma undergo periodic liver screening for focal liver detection, usually with ultrasonography (US) with MRI being used when US is equivocal. Finally, contrast-enhanced MRI with extra-cellular gadolinium chelates is preferred for characterisation of indeterminate hepatic masses with liver biopsy used when tissue diagnosis is needed.
Keywords: Liver cancer imaging, computed tomography, magnetic resonance imaging, ultrasonography, positron emission tomography
Introduction
Liver imaging is commonly undertaken in patients with cancer history because, after lymph nodes, the liver is the most frequently involved organ by metastases [1]. Liver metastases most often arise from primary tumours in colon, breast, lung, pancreas and stomach [2]. Although in Europe and the United States a malignant liver mass is more likely to represent a metastatic deposit than a primary hepatic malignancy [1], hepatocellular carcinoma (HCC) is the most common primary cancer of the liver and its incidence has increased in Japan and portions of the developing world, arising mainly in patients with chronic liver disease [3]. In both situations, accurate detection of malignant liver disease remains crucial to patient management [4]. However, since benign liver lesions are very common [5, 6], liver-imaging strategies should incorporate liver lesion characterisation as an equally important goal. Several imaging modalities are now available for detection and characterisation of focal liver lesions. These include ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET). Since technological advances in these modalities and associated contrast media continue to occur, no broad consensus exists over which modality should be used.
In this paper we outline a practical approach for liver imaging in the oncologic population.
Imaging hepatic metastases
Survey examinations
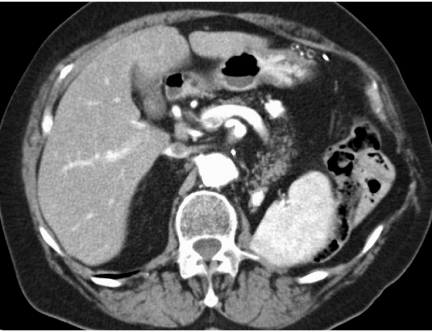
Patients with a known extra-hepatic malignancy frequently undergo abdominal survey examinations to look for liver metastases, lymph node involvement and local involvement. During liver evaluation, the primary goal is to determine the presence/absence of hepatic metastases and provide a gross estimate of liver tumour burden. Such survey examinations are best undertaken with a contrast-enhanced CT study since CT has high sensibility (93%) and specificity (100%) for detecting hepatic metastases [7]. While US and MRI also have similar accuracy, CT is preferred because it out-performs US and MRI for evaluating the extra-hepatic abdomen [8]. Other benefits of CT are easy access due to wide availability and patient-friendly protocols allowing even a chest–abdomen–pelvis CT examination in a less then 20-s breath hold using multidetector CT technology [8, 9]. In most patients portal-venous phase imaging is sufficient for examination of the abdomen and pelvis. However, in patients with vascular tumours such as neuroendocrine tumours, melanoma and renal cell cancer (Fig. 1), arterial phase imaging should be added to the portal venous phase acquisition [10]. If radiographic contrast media cannot be administered due to iodine allergy or renal insufficiency, the accuracy of CT is poor and an MRI should be performed to fully evaluate the liver. Similarly, MRI should be performed in the presence of fatty infiltration of the liver since liver metastases can be obscured when hepatic steatosis is present [11].
Figure 1.
Arterial phase contrast-enhanced computed tomography in a patient with renal cell carcinoma demonstrating hypervascular metastases to the pancreas.
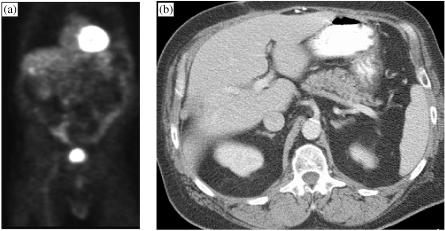
The role of fluorine-18-labelled fluorodeoxyglucose (FDG)–PET imaging for abdominal evaluation in patients with colon rectal cancer, ovarian cancer and other cancers is still under evaluation [12–14]. If available, a combined PET–CT examination is the preferred approach since it allows anatomic localisation of ‘hot-spots’ for characterisation [15]. However, given the relatively higher cost of PET and its limited availability [15], at present the use of PET or PET–CT for routine abdominal survey examination is not a practical consideration. Furthermore, regarding liver metastases detection, in our experience the major drawback of PET is that the heterogeneous hepatic uptake of the FDG radiopharmaceutical makes it difficult to exclude small liver metastases (Fig. 2(a, b)) [15]. Indeed, our experience has demonstrated a higher accuracy for contrast-enhanced MRI over PET (100 vs. 93.7%) [16]. However, there is an important role for PET at the time of initial diagnosis for cancer staging, primarily due to its higher sensitivity for detecting extra-hepatic foci of metastases in areas in which CT has sub-optimal sensitivity, such as metastases in non-enlarged lymph nodes [15].
Figure 2.
PET (a) demonstrates a focus of uptake in the right liver lobe in a patient with colon carcinoma who had undergone a metastasectomy, as seen on the CT (b). No tumour recurrence was present on follow-up scans.
Staging examinations
In contrast to survey examinations, liver staging examinations are performed in patients with known hepatic malignancy (primary or secondary) when liver resection is being considered. In this setting accurate detection of individual liver metastases is important because their number and location determine whether the patient is a suitable candidate for surgical therapy [17]. In this setting, our approach is to perform an MRI examination using liver-specific contrast media. Several studies have consistently shown the superiority of a liver-specific contrast agent-enhanced MRI examination over contrast-enhanced CT or a non-contrast MRI examination [18]. For example, Sahani et al. demonstrated that combined pre- and post-contrast gadoxetate MRI had improved sensitivity for liver lesion detection compared to pre-contrast MRI, and similar sensitivity to dual-phase CT and fewer false-positive lesions identified with pre- and post-contrast gadoxetate MRI compared to dual-phase helical CT [19]. Indeed, as a result, computed tomography during arterial portography is no longer needed for accurate staging studies.
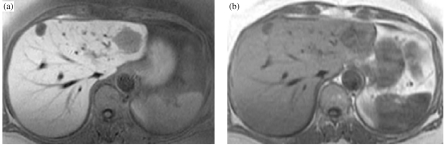
Currently, several liver-specific compounds are available for liver MRI. These agents include water-soluble paramagnetic chelates with hepatocyte uptake (mangafodipir, gadobenate, gadoxetate) and reticuloendothelial system compounds (ferumoxide, ferucarbotran) [18]. After intravenous administration, iron oxide particles are taken up by the reticuloendothelial system. Their primary effect on MR images is to decrease signal intensity of normal areas of liver on T2-weighted images [20]. In comparison, liver metastases without reticuloendothelial function show an unchanged signal [20]. This increases the difference in signal between normal liver and liver metastases, resulting in improved detection of hepatic metastases [21–25]. However, our preference is for the hepatocyte-specific T1 contrast agents which selectively affect the signal intensity of normal liver, providing images of excellent signal-to-noise ratio by means of fast imaging sequences (Fig. 3(a, b)) [18]. In comparison, iron oxide-enhanced MRI depends on susceptibility effects [26] and produces signal intensity loss on T2-weighted images [20], and makes images particularly susceptible to motion artifacts. Furthermore, the hepatocyte-targeted compounds are better tolerated then the iron oxides [27]. Other benefits of liver-specific contrast-enhanced MRI are that dynamic images are not necessary nor does the whole liver need to be covered in a single breath hold [27].
Figure 3.
T1-weighted MRI of the liver demonstrates higher metastases–liver contrast on hepatocyte-specific contrast agent (mangafodipir) enhanced images (a) compared to the pre-contrast images (b). Note that an additional lesion is seen on post-contrast image in segment IV.
Liver lesion characterisation
As mentioned before, due to the high prevalence of benign hepatic tumours such as cysts, haemangiomas and focal nodular hyperplasia, liver lesion characterisation is as important as lesion detection. A portal phase-only contrast-enhanced CT is able to characterise the vast majority of haemangiomas [28–30]. With delayed- or equilibrium-phase scans, cysts can be readily characterised as well. However, when a lesion is indeterminate on contrast-enhanced CT a dynamic contrast-enhanced MRI with gadolinium chelates is appropriate for lesion characterisation [31]. In clinical practice, the most commonly used contrast agents are the non-specific extra-cellular gadolinium chelates because they are inexpensive, safe, and well tolerated by patients, and they can enable characterisation of a wide range of hepatic diseases [32]. Optimal imaging technique for lesion characterisation requires imaging in arterial, portal and equilibrium phases [33].
For patients undergoing staging studies using liver-specific contrast agent-enhanced MRI, a dynamic scan with gadolinium chelates can be done immediately afterwards with a second injection, resulting in a dual contrast study [34].
Imaging hepatocellular carcinoma
Hepatocellular carcinoma is the most common primary malignant hepatic neoplasm, and usually occurs in patients with chronic hepatic parenchymal disease [35]. An accurate diagnosis of HCC with cirrhosis is important for patient care and treatment decision [35]. Patients with chronic liver disease such as hepatitis C are at risk for developing HCC and undergo periodic liver screening for focal liver detection. Screening with α-fetoprotein and US is a useful tool for early diagnosis of HCC [36].
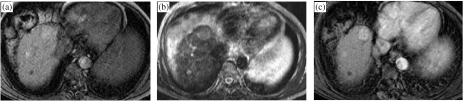
Detection of focal masses within a cirrhotic liver is a daunting challenge. In addition, distinction of HCC from other solid lesions such as regenerating nodules, dysplastic nodules and confluent hepatic fibrosis is an equally important and difficult task [35]. Ultrasonography is the primary screening test since it allows for a quick and cost-effective way to examine the liver parenchyma and can be done as frequently as needed, typically every 3–6 months [37]. However, US-based screening for HCC has a sub-optimal sensitivity and specificity, especially when liver cirrhosis is present [38]. Hence patients with an abnormal liver US showing cirrhosis or focal mass often undergo a contrast-enhanced CT or MRI examination [38]. Unfortunately, neither modality has adequate sensitivity, typically being 40–70% for early HCC detection [39, 40]. With both modalities arterial phase imaging is critical for early detection of HCC [41] (Fig. 4(a–c)). In recent years the value of dual arterial phase imaging has also been evaluated and is under investigation [42, 43].
Figure 4.
MRI of the liver in a patient with chronic liver disease. T1-weighted pre-contrast injection (a), T2-weighted (b) and arterial phase extracellular Gd-chelate-enhanced MRI (c) demonstrating a T1 and T2 hyperintense mass which is enhanced in the arterial phase, indicative of an HCC.
Nevertheless, arterially enhancing lesions greater than 1 cm are likely to be malignant but should be distinguished from arterial shunts. Since malignant lesions are typically T2 hyperintense, our preference is to use MRI because it has superior diagnostic accuracy to CT [38]. MR can also characterise regenerative nodules which are under 1 cm and T2 hypointense and dysplastic nodules which typically are greater than 1 cm, T2 hypointense and T1 hyperintense [35]. In addition, these lesions generally do not show arterial phase enhancement although exceptions do occur [44], in which case biopsy may be needed. If there is no access to a high-quality MRI facility, contrast-enhanced CT may be undertaken with a hepatic arterial phase and portal phase scans.
Summary
The goal of liver imaging in oncologic patients includes liver tumour detection and characterisation. Patients with extra-hepatic malignancy undergo survey examinations to exclude the presence of hepatic and extra-hepatic metastases and to evaluate the extent of local involvement. This metastasis survey should be done with contrast-enhanced CT, MRI being reserved for those patients unable to receive intravenous contrast or with a fatty liver. Patients with hepatic metastases being considered for metastasectomy undergo a staging examination usually with contrast-enhanced MRI using tissue-specific contrast agents. Patients with chronic liver disease at risk for developing hepatocellular carcinoma undergo periodic liver screening with US, and extra-cellular gadolinium chelate contrast-enhanced MRI is used for evaluating patients with an abnormal US. Finally, preoperative mapping of the hepatic artery, portal vein and the hepatic vein anatomy is often undertaken before surgery in patients with hepatic malignancy [45, 46]. Vascular anatomy imaging can be done with CT angiography or MR angiography, and generally requires a separate examination.
References
- 1. Khan A N, Macdonald S, Amin Z, Liver, metastasis. http://www.emedicine.com/radio/topic394.htm. November 21, 2003
- 2.Ackerman NB, Lien WM, Kondi ES, et al. The blood supply of experimental liver metastases. Distribution of hepatic artery and portal vein blood to ‘small’ liver metastases. Distribution of hepatic artery and portal vein blood to ‘small’ and ‘large’ tumors. Surgery. 1969;66:1067–72. [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 4.Semelka RC, Worawattanakul S, Kelekis NL , et al. Liver lesion detection, characterization, and effect on patient management: comparison of single-phase spiral CT and current MR techniques. J Magn Reson Imaging. 1997;7:1040–7. doi: 10.1002/jmri.1880070616. [DOI] [PubMed] [Google Scholar]
- 5.Karhunen PJ. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol. 1986;39:183–8. doi: 10.1136/jcp.39.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noone TC, Semelka RC, Balci NC, Graham ML. Common occurrence of benign liver lesions in patients with newly diagnosed breast cancer investigated by MRI for suspected liver metastases. J Magn Reson Imaging. 1999;10:165–9. doi: 10.1002/(sici)1522-2586(199908)10:2<165::aid-jmri9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Chezmar JL, Rumancik WM, Megibow AJ, Hulnick DH, Nelson RC, Bernardino ME. Liver and abdominal screening in patients with cancer: CT versus MR imaging. Radiology. 1988;168:43–7. doi: 10.1148/radiology.168.1.3380982. [DOI] [PubMed] [Google Scholar]
- 8.Hopper KD, Singapuri K, Finkel A. Body CT and oncologic imaging. Radiology. 2000;215:27–40. doi: 10.1148/radiology.215.1.r00ap1727. [DOI] [PubMed] [Google Scholar]
- 9.Kulinna C, Helmberger T, Kessler M, Reiser M. Improvement in diagnosis of liver metastases with the multi-detector CT. Radiologe. 2001;41:16–23. doi: 10.1007/s001170050923. [DOI] [PubMed] [Google Scholar]
- 10.Baron RL. Understanding and optimizing use of contrast material for CT of the liver. Am J Roentgenol. 1994;163:323–31. doi: 10.2214/ajr.163.2.8037023. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Wu N, Ouyang H. Imaging manifestations of tumors metastasized in fatty liver. Zhonghua Zhong Liu Za Zhi. 1998;20:132–4. [PubMed] [Google Scholar]
- 12.Goldberg MA, Lee MJ, Fischman AJ, Mueller PR, Alpert NM, Thrall JH. Fluorodeoxyglucose PET of abdominal and pelvic neoplasms: potential role in oncologic imaging. Radiographics. 1993;13:1047–62. doi: 10.1148/radiographics.13.5.8210589. [DOI] [PubMed] [Google Scholar]
- 13.Tutt AN, Plunkett TA, Barrington SF, Leslie MD. The role of positron emission tomography in the management of colorectal cancer. Colorectal Dis. 2004;6:2–9. doi: 10.1111/j.1463-1318.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 14.Pannu HK, Bristow RE, Cohade C, Fishman EK, Wahl RL. PET-CT in recurrent ovarian cancer: initial observations. Radiographics. 2004;24:209–23. doi: 10.1148/rg.241035078. [DOI] [PubMed] [Google Scholar]
- 15.Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using non-invasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748–56. doi: 10.1148/radiol.2243011362. [DOI] [PubMed] [Google Scholar]
- 16.Kalva S, Sahani D, Ryan D, Fishman A, Hahn P, Saini S. RSNA ’03 Gastrointestinal Session, Scientific Papers. Radiological Society of North America; Detection of liver metastases from GI cancer: comparison of high-resolution Mn-DPDP contrast-enhanced MRI and FDG–PET. [Google Scholar]
- 17.Solbiati L, Cova L, Ierace T, Marelli P, Dellanoce M. Liver cancer imaging: the need for accurate detection of intrahepatic disease spread. J Comput Assist Tomogr. 1999;23(Suppl. 1):S29–37. doi: 10.1097/00004728-199911001-00005. [DOI] [PubMed] [Google Scholar]
- 18.Weinmann HJ, Ebert W, Misselwitz B, Schmitt-Willich H. Tissue-specific MR contrast agents. Eur J Radiol. 2003;46:33–44. doi: 10.1016/s0720-048x(02)00332-7. [DOI] [PubMed] [Google Scholar]
- 19.Sahani D, Bluemke D, Shamsi K, Breuer J, Belzer T, Saini S. RSNA ’03 Gastrointestinal Session, Scientific Papers. Radiological Society of North America; MR imaging using a liver specific contrast agent (gadolinium-EOB-DTPA): US multicenter phase III study of efficacy and safety. [Google Scholar]
- 20.Bluemke DA, Weber TM, Rubin D, et al. Hepatic MR imaging with ferumoxides: multicenter study of safety and effectiveness of direct injection protocol. Radiology. 2003;228:457–64. doi: 10.1148/radiol.2282012061. [DOI] [PubMed] [Google Scholar]
- 21.Bluemke DA, Paulson EK, Choti MA, DeSena S, Clavien PA. Detection of hepatic lesions in candidates for surgery: comparison of ferumoxides-enhanced MR imaging and dual-phase helical CT. Am J Roentgenol. 2000;175:1653–8. doi: 10.2214/ajr.175.6.1751653. [DOI] [PubMed] [Google Scholar]
- 22.Kondo H, Kanematsu M, Hoshi H, et al. Preoperative detection of malignant hepatic tumors: comparison of combined methods of MR imaging with combined methods of CT. Am J Roentgenol. 2000;174:947–54. doi: 10.2214/ajr.174.4.1740947. [DOI] [PubMed] [Google Scholar]
- 23.Reimer P, Tombach B. Hepatic MRI with SPIO: detection and characterization of focal liver lesions. Eur Radiol. 1998;8:1198–204. doi: 10.1007/s003300050535. [DOI] [PubMed] [Google Scholar]
- 24.Ros PR, Freeny PC, Harms SE , et al. Hepatic MR imaging with ferumoxides: a multicenter clinical trial of the safety and efficacy in the detection of focal hepatic lesions. Radiology. 1995;196:481–8. doi: 10.1148/radiology.196.2.7617864. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz LH, Seltzer SE, Tempany CM , et al. Superparamagnetic iron oxide hepatic MR imaging: efficacy and safety using conventional and fast spin-echo pulse sequences. J Magn Reson Imaging. 1995;5:566–70. doi: 10.1002/jmri.1880050516. [DOI] [PubMed] [Google Scholar]
- 26.Ward J, Guthrie JA, Wilson D, et al. Colorectal hepatic metastases: detection with SPIO-enhanced breath-hold MR imaging—comparison of optimized sequences. Radiology. 2003;228:709–18. doi: 10.1148/radiol.2283020376. [DOI] [PubMed] [Google Scholar]
- 27.Semelka RC, Helmberger TK. Contrast agents for MR imaging of the liver. Radiology. 2001;218:27–38. doi: 10.1148/radiology.218.1.r01ja2427. [DOI] [PubMed] [Google Scholar]
- 28.Van Hoe L, Baert AL, Gryspeerdt S, et al. Dual-phase helical CT of the liver: value of an early-phase acquisition in the differential diagnosis of noncystic focal lesions. Am J Roentgenol. 1997;168:1185–92. doi: 10.2214/ajr.168.5.9129409. [DOI] [PubMed] [Google Scholar]
- 29.Yun EJ, Choi BI, Han JK , et al. Hepatic hemangioma: contrast-enhancement pattern during the arterial and portal venous phases of spiral CT. Abdom Imaging. 1999;24:262–6. doi: 10.1007/s002619900492. [DOI] [PubMed] [Google Scholar]
- 30.Horton KM, Bluemke DA, Hruban RH, Soyer P, Fishman EK. CT and MR imaging of benign hepatic and biliary tumors. Radiographics. 1999;19:431–51. doi: 10.1148/radiographics.19.2.g99mr04431. [DOI] [PubMed] [Google Scholar]
- 31.Oudkerk M, Torres CG, Song B, et al. Characterization of liver lesions with mangafodipir trisodium-enhanced MR imaging: multicenter study comparing MR and dual-phase spiral CT. Radiology. 2002;223:517–24. doi: 10.1148/radiol.2232010318. [DOI] [PubMed] [Google Scholar]
- 32.Pastor CM, Planchamp C, Pochon S, et al. Kinetics of gadobenate dimeglumine in isolated perfused rat liver: MR imaging evaluation. Radiology. 2003;229:119–25. doi: 10.1148/radiol.2291020726. [DOI] [PubMed] [Google Scholar]
- 33.Saini S, Nelson RC. Technique for MR imaging of the liver. Radiology. 1995;197:575–7. doi: 10.1148/radiology.197.3.7480718. [DOI] [PubMed] [Google Scholar]
- 34.Kubaska S, Sahani DV, Saini S, Hahn PF, Halpern E. Dual contrast-enhanced magnetic resonance imaging of the liver with superparamagnetic iron oxide followed by gadolinium for lesion detection and characterization. Clin Radiol. 2001;56:410–5. doi: 10.1053/crad.2000.0673. [DOI] [PubMed] [Google Scholar]
- 35.Harisinghani MG, Hahn PF. Computed tomography and magnetic resonance imaging evaluation of liver cancer. Gastroenterol Clin North Am. 2002;31:759–76. doi: 10.1016/s0889-8553(02)00028-6. [DOI] [PubMed] [Google Scholar]
- 36.Youk CM, Choi MS, Paik SW , et al. Early diagnosis and improved survival with screening for hepatocellular carcinoma. Taehan Kan Hakhoe Chi. 2003;9:116–23. [PubMed] [Google Scholar]
- 37.Nicolau C, Bianchi L, Vilana R. Gray-scale ultrasound in hepatic cirrhosis and chronic hepatitis: diagnosis, screening, and intervention. Semin Ultrasound CT MR. 2002;23:3–18. doi: 10.1016/s0887-2171(02)90026-0. [DOI] [PubMed] [Google Scholar]
- 38.Vogl TJ, Eichler K, Zangos S, Mack M, Hammerstingl R. Hepatocellular carcinoma: role of imaging diagnostics in detection, intervention and follow-up. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 2002;174:1358–68. doi: 10.1055/s-2002-35349. [DOI] [PubMed] [Google Scholar]
- 39.Libbrecht L, Bielen D, Verslype C, et al. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749–61. doi: 10.1053/jlts.2002.34922. [DOI] [PubMed] [Google Scholar]
- 40.Baron RL, Peterson MS. From the RSNA refresher courses: screening the cirrhotic liver for hepatocellular carcinoma with CT and MR imaging: opportunities and pitfalls. Radiographics. 2001;21(Spec No):S117–32. doi: 10.1148/radiographics.21.suppl_1.g01oc14s117. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita Y, Mitsuzaki K, Yi T, et al. Small hepatocellular carcinoma in patients with chronic liver damage: prospective comparison of detection with dynamic MR imaging and helical CT of the whole liver. Radiology. 1996;200:79–84. doi: 10.1148/radiology.200.1.8657948. [DOI] [PubMed] [Google Scholar]
- 42.Laghi A, Iannaccone R, Rossi P, et al. Hepatocellular carcinoma: detection with triple-phase multi-detector row helical CT in patients with chronic hepatitis. Radiology. 2003;226:543–9. doi: 10.1148/radiol.2262012043. [DOI] [PubMed] [Google Scholar]
- 43.Murakami T, Kim T, Takamura M, et al. Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology. 2001;218:763–7. doi: 10.1148/radiology.218.3.r01mr39763. [DOI] [PubMed] [Google Scholar]
- 44.Krinsky GA, Lee VS, Theise ND , et al. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445–54. doi: 10.1148/radiology.219.2.r01ma40445. [DOI] [PubMed] [Google Scholar]
- 45.Van Thiel DH, Wright HI, Fagiuoli S, Caraceni P, Rodriguez-Rilo H. Preoperative evaluation of a patient for hepatic surgery. J Surg Oncol Suppl. 1993;3:49–51. doi: 10.1002/jso.2930530514. [DOI] [PubMed] [Google Scholar]
- 46.Ozeki Y, Uchiyama T, Katayama M, Sugiyama A, Kokubo M, Matsubara N. Extended left hepatic trisegmentectomy with resection of main right hepatic vein and preservation of middle and inferior right hepatic veins. Surgery. 1995;117:715–7. doi: 10.1016/s0039-6060(95)80018-2. [DOI] [PubMed] [Google Scholar]