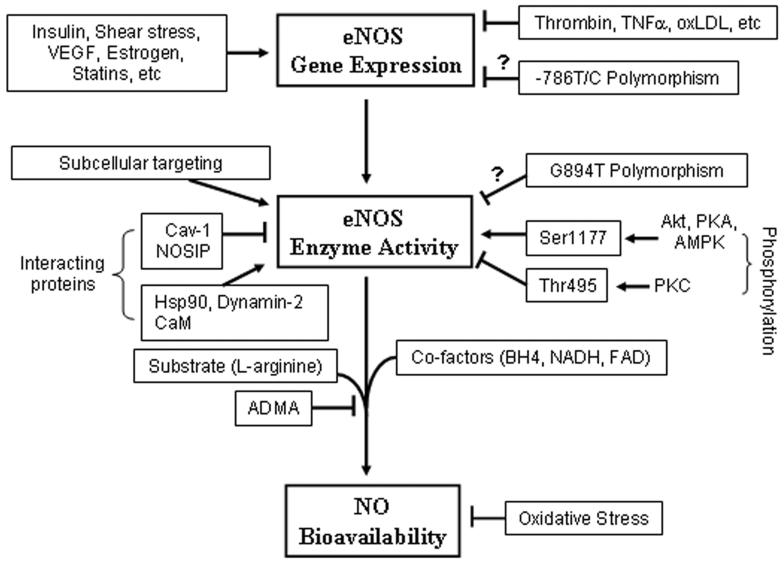
Figure 1.
Regulatory mechanisms of endothelial NO production at three different levels. While shear stress, insulin, VEGF, estrogen, and statins increase eNOS gene expression, several factors that are involved in atherosclerotic disease, such as thrombin, TNF-α and Ox-LDL, suppress eNOS gene expression. Whether the −786T/C substitution in the promoter region reduces eNOS gene expression is not clear. Subcellular targeting of eNOS regulated by co- or post-translational modifications, such as N-myristoylation and cysteine, palmitoylation is critical for optimal NO production. The mechanism by which localization influences eNOS activation is not fully understood. Interaction of eNOS with caveolin-1 (Cav-1) and NOSIP proteins reduces eNOS enzymatic activity, and interaction with heat shock protein 90 (Hsp90) and dynamin-2 increases eNOS enzymatic activity. Increase in intracellular Ca2+ concentration forms the Ca2+-calmodulin (CaM) complex, which also interacts with eNOS and stimulates eNOS enzymatic activity. Whether the eNOS Glu298Asp variant (G894T polymorphism) affects eNOS enzymatic activity remains obscure. Phosphorylation of human eNOS at serine 1177 (Ser1177) by protein kinases Akt, PKA or AMPK enhances, whereas phosphorylation of the enzyme at threonine 495 (Thr495) by PKC inhibits eNOS enzymatic activity. The eNOS enzymatic activity is also dependent on the availability of co-factors, such as BH4, NADH, FAD, and substrate L-arginine. Increase in production of endogenous ADMA reduces NO production. Increase in oxidative stress inactivates NO resulting in decreased NO bioavailability.