Fig. 1.
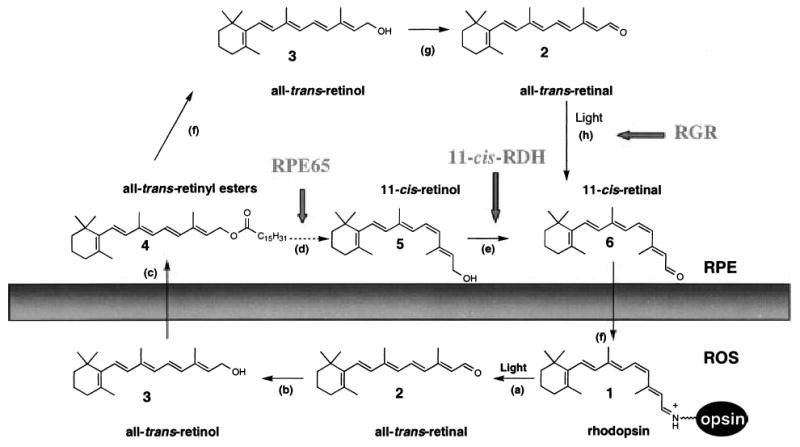
Chemistry of the retinoid cycle reactions in the vertebrate retina. The retinoid cycle reactions were reviewed recently (McBee et al. 2001a). In the rod outer segment (ROS), light causes the isomerization (reaction a) of rhodopsin chromophore, 11-cis-retinylidene (1), to all-trans-retinylidene. All-trans-retinal (2) is hydrolyzed and then reduced (reaction b) in the reaction catalyzed by all-trans-retinal-specific RDH(s). All-trans-retinol (3) diffuses to RPE where it is esterified by LRAT (reaction c) to retinyl esters (4). All-trans-retinyl esters can be hydrolyzed by a yet unidentified retinyl hydrolase (reaction f) generating all-trans-retinol. Next, the pathway branches and all-trans-retinol, or its derivative, is isomerized to 11-cis-retinol (5) in a reaction that involves an abundant RPE protein, termed RPE65 (poorly defined reactions d). 11-cis-retinol is then oxidized by 11-cis-RDH (RDH5) and other dehydrogenases (Haeseleer et al. 2002) to 11-cis-retinal (6) (reaction e) to complete the cycle (modified version from (Jang et al. 2000)). An alternative branch involves oxidation of all-trans-retinol to all-trans-retinal (reaction g) and photoisomerization by RGR of the aldehyde to 11-cis-retinal (reaction h). 11-cis-retinal diffuses back to ROS, where it recombines with opsin to reform rhodopsin.
