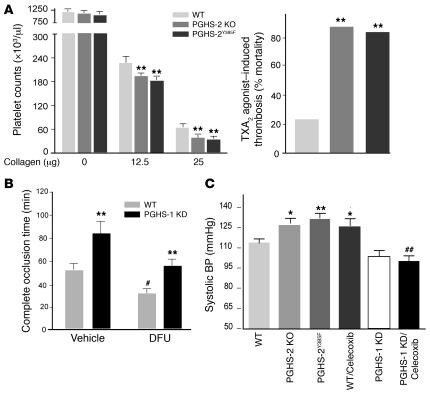
Figure 3. PGHS-2 disruption or inhibition promotes thrombogenesis and hypertension and modulation effect of PGHS-1 KD.
(A) Circulating platelets before and 2 minutes after injection of collagen (12.5 and 25 μg/kg) plus epinephrine (15 μg/ml, 100 μl) into WT, PGHS-2Y385F, and PGHS-2 KO mice on a mixed C57BL/6 × 129/Sv background. Thrombocytopenia was more pronounced (**P < 0.01) in PGHS-2–disrupted mice. Sudden death was induced more commonly (**P < 0.01) within 15 minutes of U46619 injection in PHGS-2–deleted or –mutated mice. (B) The time to thrombotic carotid artery occlusion after photochemical injury was delayed in PGHS-1 KD mice (**P < 0.01) but accelerated by DFU treatment (#P < 0.05). The time to occlusion in DFU-treated animals was delayed in the PGHS-1 KD group compared with WT controls (**P < 0.01), while the time to occlusion in DFU-treated PGHS-1 KD mice did not differ significantly from that in vehicle-treated WT controls on a mixed C57BL/6 × 129/Sv background. (C) Systolic blood pressure, as measured by the tail cuff method, was elevated significantly in 3-month-old PGHS-2 KO, PGHSY385F, and celecoxib-treated (100 mg/kg/d for 30 days) mice as compared with WT mice on a mixed C57BL/6 × 129/Sv background (*P < 0.05; **P < 0.01). The hypertensive effect of celecoxib was attenuated in PGHS-1 KD mice compared with that in WT (##P < 0.01) mice.