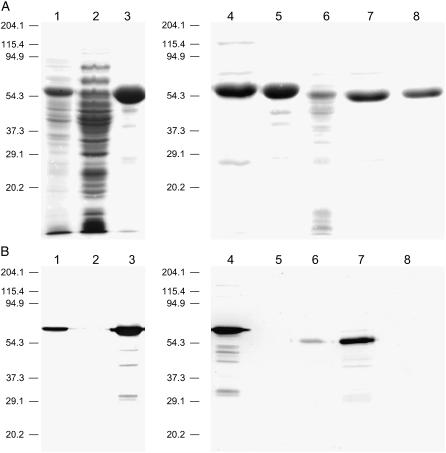
Figure 2.
Purification of heterologously expressed PCS enzymes from model plants by immobilized metal affinity chromatography. A, SDS-PAGE analysis of fractions obtained during purification of AtPCS1 and LjPCS1. Gels were stained conventionally with Coomassie Brilliant Blue R-250. Lanes: 1, desalted crude extract of recombinant AtPCS1; 2, proteins eluted with 50 mm imidazole; 3, AtPCS1 eluted with 250 mm imidazole; 4, AtPCS1 preparation used to obtain 5, prior to thrombin digestion; 5, AtPCS1 after tag removal with thrombin; 6, desalted crude extract of recombinant LjPCS1; 7, LjPCS1 eluted with 250 mm imidazole; and 8, LjPCS1 after tag removal with thrombin. Lanes were loaded with 20 μg of protein. B, Immunoblot analysis of fractions shown in Figure 2A, using anti-polyHis monoclonal antibody and an alkaline phosphatase-based detection system (Sigma). Lanes were loaded with 2 μg of protein.