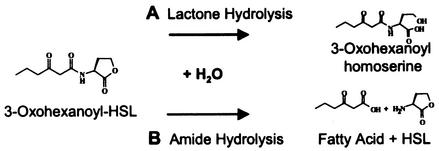
FIG. 1.
Mechanisms by which a model acyl-HSL quorum signal can be chemically or biochemically degraded. (A) Hydrolytic cleavage of the lactone ring yields the corresponding acyl-homoserine. Chemically, this occurs at rates influenced by half-life kinetics and pH; biochemically, it occurs via the activity of acyl-HSL lactonases encoded by diverse bacteria (see the text). (B) Amide cleavage yields HSL and the corresponding fatty acid. The amide bond of acyl-HSLs is chemically stable under nonextreme temperature and pH but can be cleaved by an acyl-HSL acylase encoded by the bacterium V. paradoxus.