Fig. 5.
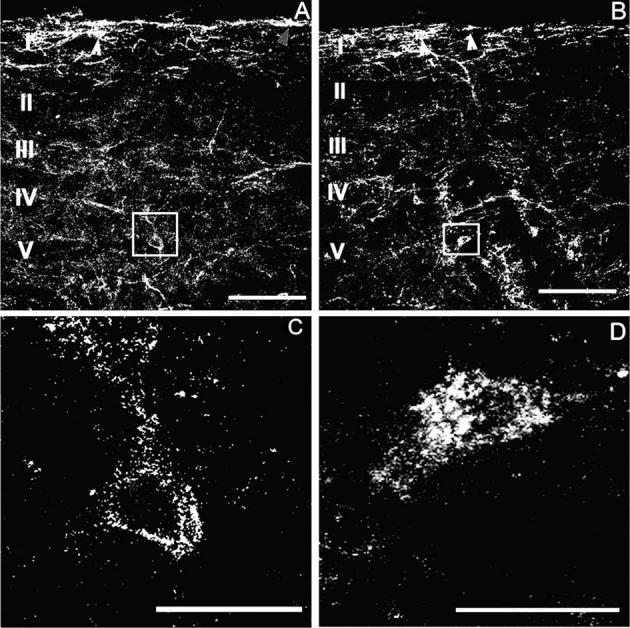
Sustained morphine-induced NK-1 receptor internalization in deep dorsal horn neurons. Confocal images show that mechanical stimulation (2 min pinch) induced NK-1 receptor internalization in the spinal cord dorsal horn of rats 6 days after placebo (A, C) or morphine (B, D) pellets were implanted subcutaneously. NK-1 receptor internalization after mechanical stimulation is confined to lamina I spinal neurons in placebo-pelleted animals (A). Arrowheads indicate neurons with NK-1 receptor internalization in lamina I spinal neurons. In contrast, sustained exposure to morphine induces NK-1 receptor internalization in deeper laminae III–V neurons as well as in lamina I neurons (B). Arrows indicate lamina I spinal neurons with NK-1 receptor internalization. High power magnification illustrates the difference between deep dorsal horn neurons with and without NK-1 receptor internalization (C, D). Rats that were treated with morphine pellets across 6 days show neurons that have internalized the NK-1 receptor (D). The cytoplasm of these neurons contains bright, immunofluorescent NK-1 receptor-immunoreactive endosomes, defined as an intensely NK-1 receptor-immunoreactive intracellular organelle 0.1–0.7 μm in diameter that are clearly not part of the external plasma membrane (D). In placebo-treated rats, deep dorsal horn neurons contained less than five endosomes per cell (C). In the present study, we considered a cell to have internalized receptors if it contained >20 endosomes. (A) and (C) were produced by superimposition of three optical sections taken at 2.4 μm in sagittal sections of L4, the scale bar is 100 μm; (B) and (D) were produced by superimposition of seven optical sections taken at 0.6 μm at L4, the scale bar is 20 mm.
