Figure 1.
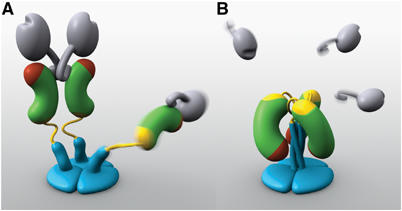
Models of two VP4 conformations. (A) The primed state. Two rigid subunits form the spike visible in electron cryomicroscopy image reconstructions of trypsin-primed virions. A third subunit is flexible. VP8* is gray, with an N-terminal tether and a globular head creased by the sialoside-binding site. The VP5* antigen domain is green bean-shape, with a red membrane interaction region and a yellow GH loop. An additional β-strand C-terminal to the antigen domain is also yellow. The spike body includes the VP5* antigen domain, part of the VP8* tether, and the GH loop. The foot is blue, as is a protruding region that rearranges into the coiled-coil. (B) The putative post-membrane penetration state. VP8* has dissociated; the yellow parts of each subunit have joined in a β-annulus; the α-helical triple coiled-coil has zipped up; and the VP5* antigen domain has folded back. The models were produced by Digizyme, Inc.
