Figure 2.
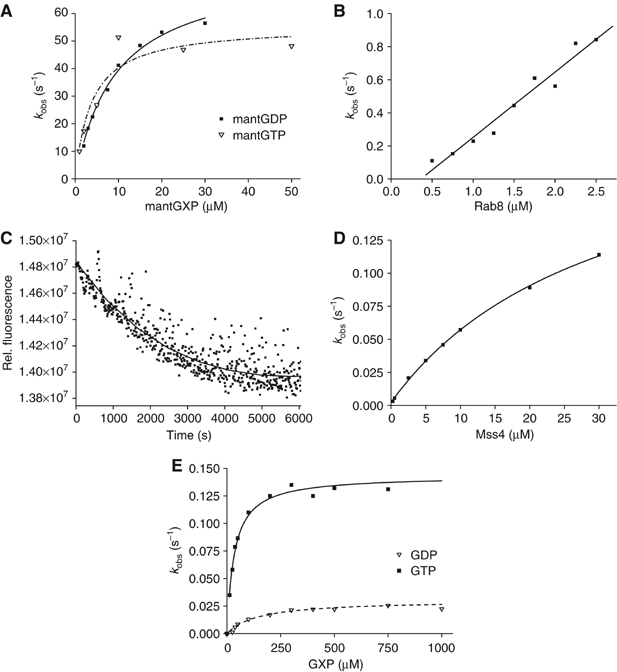
(A) Association kinetics of nucleotide-free Rab8 (0.5 μM) and different concentrations of mantGDP or mantGTP. Formation of the complex was monitored by FRET from tryptophan to the mant group in a stopped-flow apparatus. Pseudo first-order rate constants were obtained from single-exponential fits to the data. (B) Dependence of the observed rate constant for the association reaction of dansyl-labeled MSS4 (0.25 μM) on the concentration of nucleotide-free Rab8. (C) Dissociation kinetics of the dansyl-MSS4:Rab8 (1 μM) complex upon mixing with a 10-fold molar excess of unlabeled MSS4. (D) Hyperbolic fit of the MSS4-catalyzed GDP exchange rates for Rab8 (0.4 μM). The exchange reaction was monitored by FRET from tryptophan to mantGTP added to the reaction mix at 200 μM. (E) Dependence on the nucleotide concentration of the observed rate constants for the interaction of GTP or GDP with MSS4:Rab8 complex (0.4 μM). The reaction was monitored using changes in tryptophan fluorescence upon nucleotide binding.
