Figure 4.
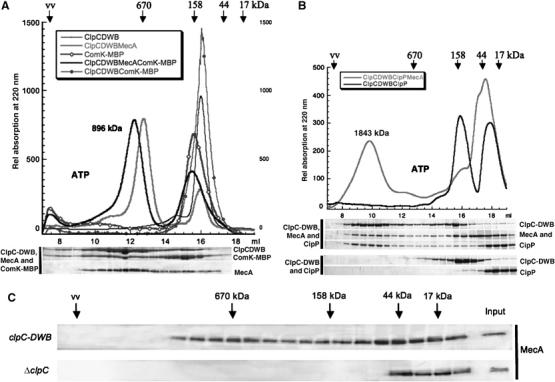
(A) The higher oligomeric complex of ClpC-DWB and MecA can bind substrate protein. The elution profile of size-exclusion chromatography experiments with ClpC-DWB (10 μM), MecA (10 μM), ComK-MBP (10 μM) (preincubated with ATP (5 mM) and 0.5 mM ATP in the running buffer) as indicated in the legend. The relative position and molecular weights of standard proteins are indicated above the elution profile. The size of the higher oligomeric complex consisting of ClpC-DWB, MecA and ComK-MBP (896±22 kDa) determined by multiangle light scattering is indicated. The fractions were analyzed as described in Figure 1 and depicted below the elution profile. (B) The ClpCP protease complex cannot assemble in the absence of MecA. The elution profile of size-exclusion chromatography experiments with ClpC-DWB (10 μM), MecA (10 μM), ClpP (10 μM) (preincubated with ATP (5 mM) and 0.5 mM ATP in the running buffer) as indicated in the legend. The relative position and molecular weights of standard proteins are indicated above the elution profile. The size of the higher oligomeric complex consisting of ClpC-DWB, MecA and ClpP (1843±10 kDa) determined by multiangle light scattering is indicated. The fractions were analyzed as described above and depicted below the elution profile. (C) Comparison of the size distribution of MecA in lysates prepared from clpC-DWB and ΔclpC B. subtilis strains. The soluble fractions of lysates of clpC-DWB and ΔclpC strains were separated by size-exclusion chromatography. The subsequent analysis by SDS–PAGE and Western blot with Anti MecA antibodies is depicted. The relative position and molecular weights of standard proteins are indicated (input is a 1:10 dilution of the lysate).
