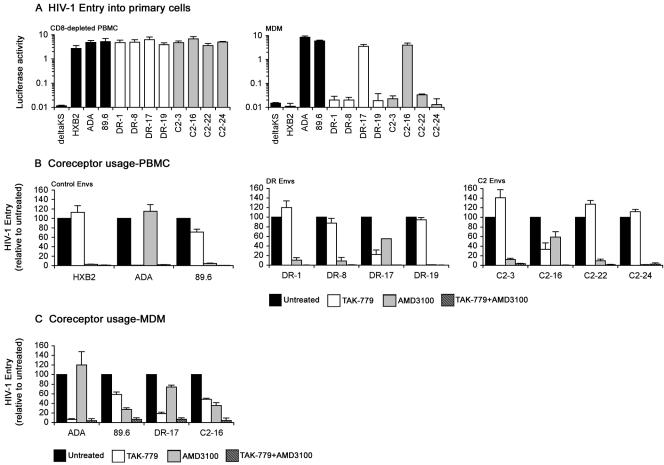
FIG. 2.
Tropism of Envs and coreceptor preference for HIV-1 entry into primary cells. (A) Single-round entry assays were conducted by infection of 0.5 × 106 phytohemagglutinin-activated, interleukin 2-stimulated PBMC that were depleted of CD8+ cells with anti-CD8-conjugated magnetic beads (Invitrogen) or infection of confluent monolayers of MDM cultured for 5 days in 48-well tissue culture plates in medium containing 10% (vol/vol) human serum and 12.5 ng/ml macrophage colony-stimulating factor that were purified from PBMC by plastic adherence with equivalent amounts of Env-pseudotyped luciferase reporter virus for 3 h at 37°C. After washing the cells to remove the virus inoculum and replacement of culture medium, luciferase activity was measured in cell lysates at 72 h postinfection (Promega). Luciferase reporter viruses pseudotyped with HXB2, ADA, 89.6, or ΔKS Env were included as controls. The data are representative of two independent experiments using cells obtained from different donors and are expressed as means from duplicate infections, and the error bars represent standard deviations. CD8-depleted PBMC (B) or MDM (C) that were prepared as described above were left untreated or preincubated with 50 nM TAK-779 (1) and/or 1.2 μM AMD3100 (10, 40) for 1 h at 37°C prior to infection with equivalent amounts of Env-pseudotyped luciferase reporter viruses containing the same concentration(s) of inhibitor. Infections proceeded for 3 h at 37°C. After washing the cells to remove the virus inoculum and replacement of culture medium, luciferase activity was measured in cell lysates at 72 h postinfection (Promega). Luciferase reporter viruses pseudotyped with ADA, HXB2, or 89.6 Env were used as controls in CD8-depleted PBMC infections, and luciferase reporter viruses pseudotyped with ADA or 89.6 Env were used as controls in MDM infections. Luciferase measurements were normalized to the values obtained in untreated infections, which were set at 100. The data are representative of two independent experiments using cells obtained from different donors and are expressed as means from duplicate infections, and the error bars represent standard deviations.