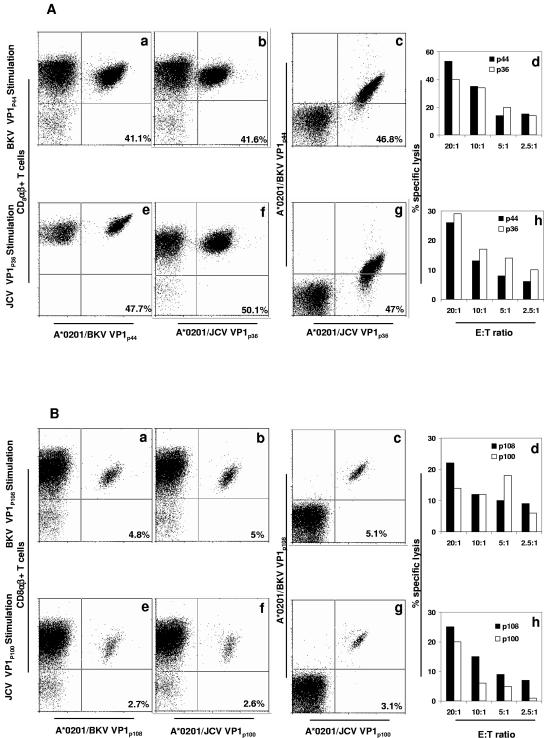
FIG. 6.
Cross-staining and cross-killing between BKV VP1 peptide-stimulated CD8+ T lymphocytes and JCV VP1 peptide-stimulated CD8+ T lymphocytes in PBMC of two HLA-A*0201+ healthy individuals. (A) Cross-reactivity between BKV VP1p44 and JCV VP1p36 peptides in study subject 8. In cross-staining, both BKV VP1p44 and JCV VP1p36 tetramers bind to a population of CD8αβ+ T lymphocytes stimulated with either VP1p44 or JCV VP1p36 peptides. The percentages of all CD8 αβ+ T cells that bind to these tetramers are indicated in each frame. An equal percentage of BKV VP1p44-stimulated CD8 αβ+ T lymphocytes was stained with the BKV VP1p44 tetramer (frame a) or the JCV VP1p36 tetramer (frame b). A double staining experiment showed that the majority of these cells stained with both tetramers (frame c). The mirror experiment is shown in frames e to g. An equal percentage of JCV VP1p36-stimulated CD8 αβ+ T lymphocytes was stained with the BKV VP1p44 tetramer (frame e) or the JCV VP1p36 tetramer (frame f). A double staining experiment showed that the majority of these cells stained with both tetramers (frame g). In a cross-killing experiment, both BKV VP1p44- and JCV VP1p36-stimulated PBMC lyse autologous target cells pulsed with either peptide. BKV VP1p44-stimulated PBMC induced lysis of BKV VP1p44-pulsed target cells more efficiently than JCV VP1p36-pulsed target cells (frame d). Conversely, JCV VP1p36-stimulated cells induced a greater lysis of JCV VP1p36-pulsed target cells than BKV VP1p44-pulsed target cells (frame h). (B) Cross-reactivity between BKV VP1p108 and JCV VP1p100 peptides in study subject 4. In cross-staining experiments, both BKV VP1p108 and JCV VP1p100 tetramers bind to a population of CD8αβ+ T lymphocytes stimulated with either BKV VP1p108 or JCV VP1p100 peptides. The percentages of all CD8 αβ+ T cells that bind to these tetramers are indicated in each frame. An equal percentage of BKV VP1p108-stimulated CD8 αβ+ T lymphocytes was stained with the BKV VP1p108 tetramer (frame a) or the JCV VP1p100 tetramer (frame b). A double staining experiment showed that the majority of these cells stained with both tetramers (frame c). A mirror experiment is shown in frames e to g. An equal percentage of JCV VP1p100-stimulated CD8 αβ+ T lymphocytes was stained with the BKV VP1p108 tetramer (frame e) or the JCV VP1p100 tetramer (frame f). A double staining experiment showed that the majority of these cells stained with both tetramers (frame g). In a cross-killing experiment, both BKV VP1p108- and JCV VP1p100-stimulated PBMC lyse autologous target cells pulsed with either peptide. As expected, BKV VP1p108-stimulated PBMC induced lysis of BKV VP1p108-pulsed target cells more efficiently than JCV VP1p100-pulsed target cells (frame d). Interestingly, JCV VP1p100-stimulated cells induced a greater lysis of BKV VP1p108-pulsed target cells than JCV VP1p100-pulsed target cells (frame h). Flow cytometry experiments were performed with PE-tagged tetramers (A, frames a, b, and f; B, frames a, b, e, and f), or APC-tagged tetramer (A, frame e). Double staining experiments were performed with a combination of PE- and APC-tagged tetramers (A, frames c and g; B, frames c and g).