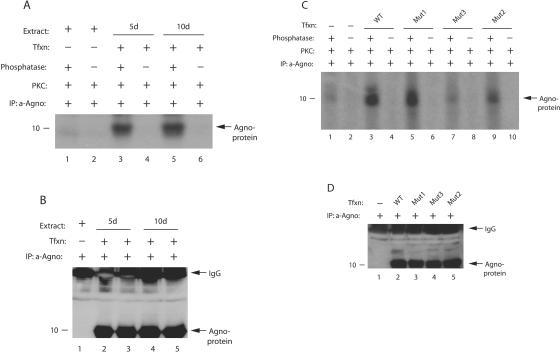
FIG. 2.
Agnoprotein is phosphorylated in vivo. (A) Phosphatase-treated agnoprotein exhibits higher signal upon phosphorylation. Whole-cell lysates were prepared from SVG-A cells, transfected with JCV Mad-1 genome, in an extraction buffer supplemented with a PhosphoSafe extraction reagent (Novagen, catalog no. 71296-3), at 5 and 10 days posttransfection. Extract (200 μg) was immunoprecipitated with an anti-agnoprotein antibody (α-Agno, 4 μl) as indicated. Whole-cell lysates were also prepared from untransfected cells and processed as described for the transfected cells. A small portion of the samples (one-sixth portion) was then either treated (lanes 1, 3, and 5) or not treated (lanes 2, 4, and 6) with phosphatase and rephosphorylated with [γ-32P]ATP using PKC enzyme as described in Materials and Methods. Samples were then washed extensively with reaction buffer and fractionated by SDS-15% PAGE, followed by autoradiography. Representative data are shown here. (B) A small portion (one-sixth portion) of immunoprecipitated samples was directly analyzed prior to phosphatase treatment by Western blotting with an α-Agno antibody. In lane 1, whole-cell lysate prepared from untransfected cells was immunoprecipitated by an anti-Agno antibody and loaded as a negative control. (C) Phosphorylation of Mut1, Mut2, and Mut3 by PKC. Mad-1 WT genome and its agnoprotein phosphorylation mutants (Mut1, where Thr21 was substituted for Ala; Mut2, where Ser7 and Ser11 were substituted for Ala; and Mut3, where Ser7, Ser11, and Thr21 were substituted for Ala) were transfected into SVG-A cells, and whole-cell lysates were prepared in an extraction buffer supplemented with a PhosphoSafe extraction reagent at day 5 posttransfection. Whole-cell lysate was also prepared from untransfected cells. WT and mutant agnoproteins were immunoprecipitated (200 μg of protein) with an anti-Agno antibody (4 μl). A one-sixth portion of the immunoprecipitated proteins was subjected to dephosphorylation, followed by phosphorylation by PKC, fractionation by SDS-PAGE, and analysis by autoradiography as described for panel A. The signal intensities for the bands at lanes 1, 3, 5, 7, and 9 were determined by scintillation counting. (D) A small portion (one-sixth portion) of immunoprecipitated samples was analyzed by Western blotting prior to phosphatase treatment with an α-Agno antibody. In lane 1, whole-cell lysate prepared from untransfected cells was immunoprecipitated by a anti-Agno antibody and loaded as a negative control. Tfxn, transfection; d, day; IP, immunoprecipitation.