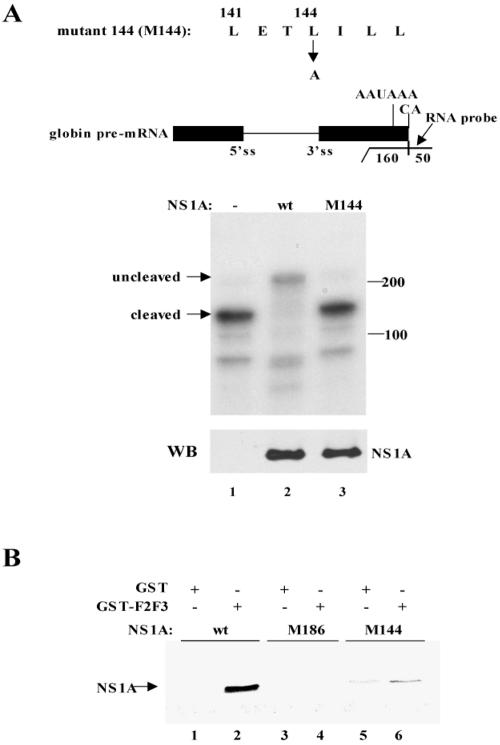
FIG. 2.
The NS1A protein containing an L-to-A substitution at position 144 did not inhibit the 3′-end processing of β-globin pre-mRNA and did not bind the F2F3 fragment of CPSF30. (A) 3′-end processing assay. 293 cells were cotransfected with a pBC12 plasmid containing a human β-globin gene and either an empty pcDNA3 plasmid (lane 1) or a pcDNA3 plasmid encoding wt NS1A protein (lane 2) or the M144 mutant NS1A protein (lane 3). The M144 sequence, which is diagrammed above the blot, was generated by RT-PCR using appropriate primers. RNA was analyzed by RNase protection using the indicated uniformly labeled RNA probe (270 nucleotides long). The protected RNA fragments were resolved by electrophoresis on a urea-polyacrylamide (5%) gel. The positions of the RNA fragments corresponding to the uncleaved and 3′-end-cleaved pre-mRNAs are indicated. No residual probe containing 270 nucleotides was detected. The amount of the wt and M144 NS1A proteins was determined by a Western blot (WB) using NS1A antibody. (B) GST pull-down assay. GST-F2F3 or GST was mixed with 35S-labeled wt, M186 mutant, or M144 mutant NS1A protein as indicated, followed by affinity chromatography on glutathione-Sepharose.