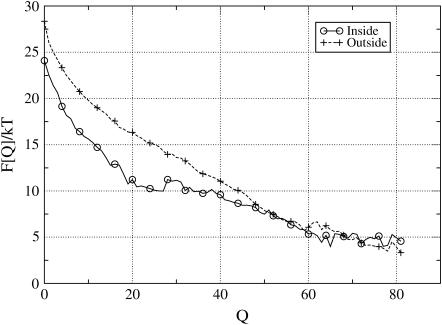
FIGURE 3.
Plots of the free energy, F(Q) at T = TF/2, as a function of the number of native contacts Q (Eq. 7) for the conformation that have at least one amino acid inside the open cage (circles), and for the one free in solution (crosses). The open cage is covered with the most attractive amino acid, Phe, with API = −0.23 kT in the rim area, whereas for the hydrophilic back surface we used Arg. For chain conformation close to the native state (Q > 45), there is not free-energy gain in the trapping, indicating that those states can easily diffuse away. However, for the nonnative states, there is a strong preference (up to 5 kT) to bind to the rim.