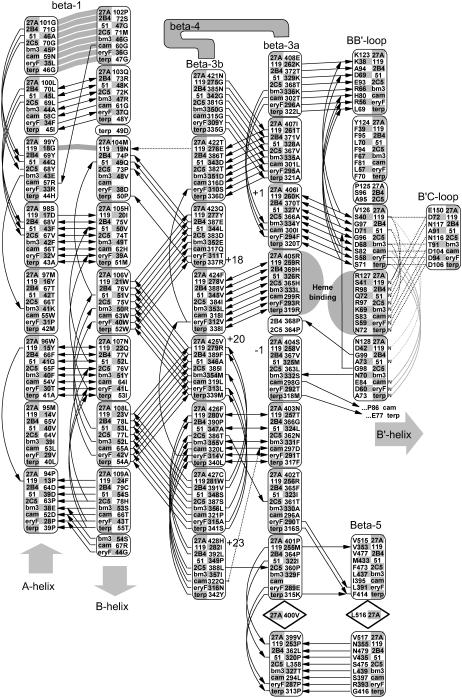
FIGURE 3.
Conserved backbone hydrogen bonding patterns in the extended β-sheet of eight selected crystal structures. As shown, there are 4–6 conserved hydrogen bonds between adjacent strands of the β-1 andβ-3 sheets. The shortened β-1 structure seen in the thermophillic archaeon CYP119 crystal structure retains the alignment of four out of five hydrogen bonds despite a four-residue truncation. Numbered positions denote distance from the heme-binding β-3 arginine or histidine-proline pair. The heme-binding residue in the β-3a strand hydrogen bonds to the heme A-ring propionate in all structures except P450Bm3, where the interaction is mediated through structural waters to the amide nitrogen and carbonyl of the adjacent residue, Ser-332. The symbols used are noted in Fig. 2.