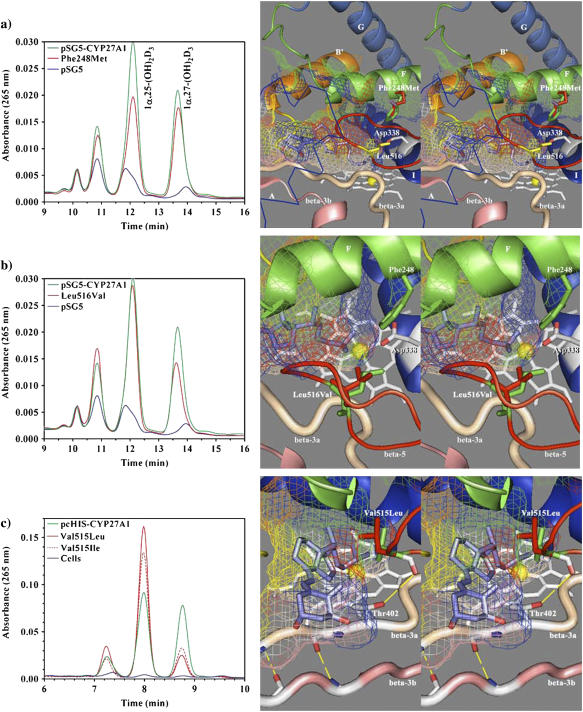
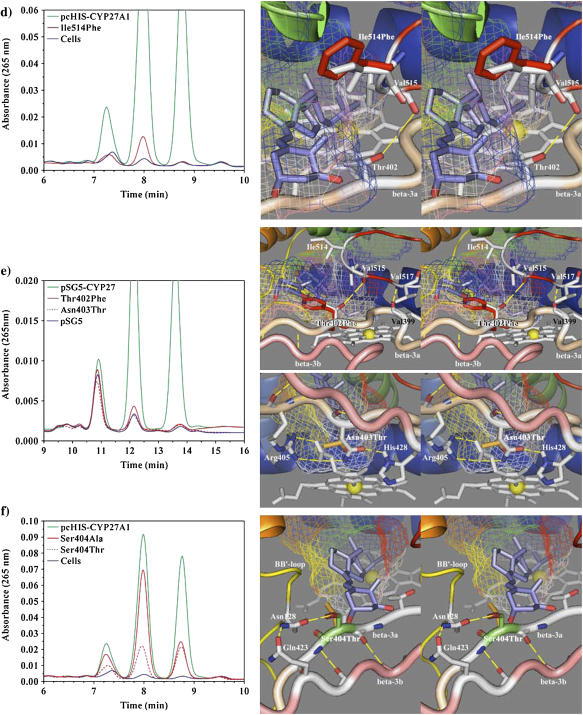
FIGURE 6.
HPLC analysis of 1α-OH-D3 metabolites from COS-1 cells transiently transfected with wild-type and site-directed mutants of CYP27A1 and stereo views of the targeted mutations with docked substrate in the binding cavity. (a) Profile of metabolites from the Phe-248-Met mutant show a preferential loss in 25-hydroxylase activity with reduced 1α,25-(OH)2D3 formation relative to 1α,27-(OH)2D3. The stereo image illustrates the position of the wild-type phenylalanine (green) and mutant methionine (red) side chain in proximity to Leu-516 (yellow) in the β-5 sheet and Asp-338 (white) in the I-helix. Contact of Phe-248 with the binding cavity is minor, suggesting that the effect of the mutation may be mediated through contact with Leu-516 in the β-5 sheet or a gating effect at Asp-338. The 1α-OH-D3 substrate is shown inside the mesh framework of the binding cavity on the heme distal face. (b) The metabolism of 1α-OH-D3 by the Leu-516-Val mutant is opposite in effect to the Phe-248-Met mutation and shows a preferential reduction in 27-hydroxylase activity and a minor increase in the formation of a 24-hydroxylated product associated with reduced steric contact with substrate. (c) The enhanced 25-hydroxylase and reduced 27-hydroxylase activities associated with the Val-515-Leu (shown in the stereo view) and Val-515-Ile mutations in the β-5 hairpin appear to result from increased steric contact with the substrate D-ring. (d) The Ile-514-Phe mutation results in a considerable loss of enzyme activity consistent with an unfavorable steric conflict with the substrate A-ring, occlusion of the substrate access channel, or a distortion of the β-5 sheet through disruption of a structural link between Thr-402 hydroxyl and Val-515 amide carbonyl. (e) The loss of activity seen with the Thr-402-Phe mutation is consistent with steric conflict with the substrate A-ring, which would impede substrate access to the binding cavity and with disruption of the β-5 sheet through the loss of a structural hydrogen bond between Thr-402 amide nitrogen and/or hydroxyl with Val-515 amide carbonyl. The loss of activity associated with the Asn-403-Thr mutation is consistent with the loss of a heme-binding interaction to the A-ring propionate, which is stabilized by His-428 and conserved in CYP27A1 and CYP27B1 species homologs. (f) Metabolic profiles seen for the Ser-404-Ala and Ser-404-Thr mutations. Disruption of a hydrogen bond to Asn-128 in the Ser-404-Ala mutant is hypothesized to alter the orientation of the B-B′ loop and disrupt bound substrate orientations leading to better retention of 27-hydroxylation. The Ser-404-Thr mutation, shown in the stereo view, maintains the contact with Asn-128, but the threonine methyl group results in a loss of enzyme activity by sterically disrupting substrate access to the binding cavity.