Figure 7.
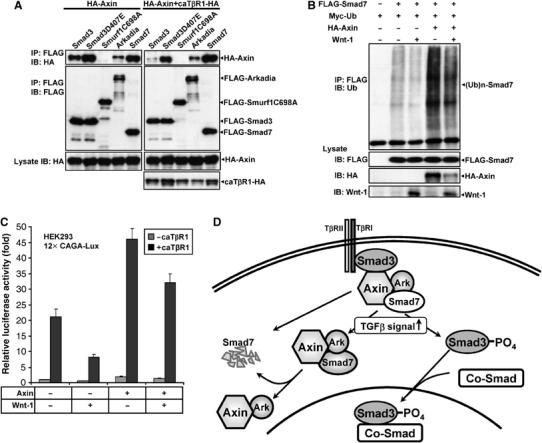
Axin constitutively associates with Arkadia and Smad7. (A) HA-Axin together with the various plasmids indicated were cotransfected with or without caTβRI into 293T cells. After 4 h treatment with MG132 (10 μM), cells were lysed and lysates were immunoprecipitated by anti-FLAG, followed by Western blotting using anti-HA and rabbit anti-FLAG. (B) Wnt-1 attenuated both basal and Axin-induced Smad7 ubiquitination. HEK293T cells were transfected with 0.5 μg of FLAG-Smad7, Myc-ubiquitin with or without 1.5 μg of HA-Axin or/and Wnt-1 as indicated. Cells were treated with MG132 (10 μM) for 4 h before lysed. Cell lysates were subjected to immunoprecipitation with anti-FLAG antibody and polyubiquitination of Smad7 was detected with anti-ubiquitin antibody. Wnt-1 in total cell lysates was probed with anti-Wnt-1. Similar results were obtained in three separate experiments. (C) Effects of Wnt-1 on the TGF-β-dependent transcriptional activity. 12 × CAGA-Lux was transfected into HEK293 cells with or without Axin, caTβRI, or/and Wnt-1 as indicated. Wnt-1 could reduce the reporter gene transcription in the presence or absence of transfected Axin. (D) Model of scaffolding roles of Axin in TGF-β signaling. Axin forms a complex with Smad3, Arkadia, and Smad7, of which Smad3 has physical contact with the type I receptor. Upon ligand stimulation, Axin promotes Smad3 phosphorylation; phosphorylated Smad3 dissociates from the Axin complex, and then combines with Smad4 to activate transcription in the nucleus. In addition, Axin acts as a scaffold to facilitate Arkadia-mediated polyubiquitination of Smad7 regardless of TGF-β signaling, leading to Smad7 degradation. Implied here is also that Axin can sequester Smad7 in the cytoplasm.
