Figure 3.
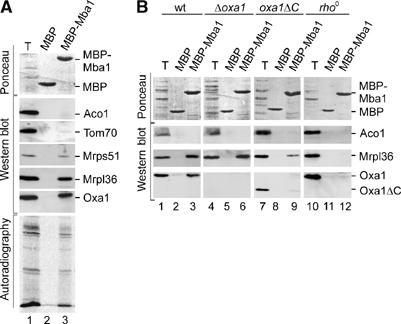
Mba1 binds mitochondrial ribosomes in vitro. (A) Recombinant MBP and MBP-Mba1 were purified and immobilized on an amylose resin. Mitochondrial translation products were radiolabeled in isolated mitochondria. Mitochondria were solubilized. The resulting extract was clarified by centrifugation and subjected to the immobilized MBP fusion proteins. Following extensive washing, bound proteins were eluted with sample buffer, resolved by SDS–PAGE and transferred to nitrocellulose. Transferred proteins were analyzed by staining with Ponceau S (upper panel) or by Western blotting with antibodies against proteins of the large (Mrpl36) and small (Mrps51) ribosomal subunit, Oxa1, aconitase (Aco1) and Tom70. The lower panel shows the radioactive translation products that were detected by autoradiography. Total lanes (T) show 10% of the mitochondrial extract that was applied to the resin. (B) Translation products were radiolabeled in mitochondria isolated from wild type (wt), a Δoxa1 mutant, a mutant containing a C-terminally truncated variant of Oxa1 (oxa1ΔC) and a rho0 strain. The binding of ribosomes and Oxa1 to the immobilized MBP-Mba1 fusion protein was assessed as in (A).
