Figure 1.
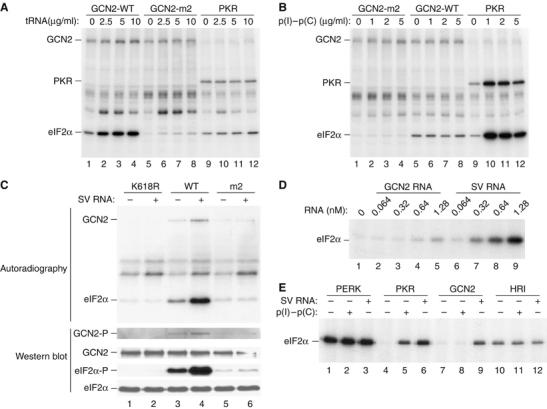
SV RNA stimulates GCN2-mediated phosphorylation of eIF2α. Purified wild-type GCN2 (GCN2-WT), the HisRS mutant (GCN2-m2) and PKR were assayed for their ability to phosphorylate eIF2α in the absence or the presence of increasing concentrations of uncharged bovine liver tRNA (Sigma) (A) or of poly(I)–poly(C) (Sigma) (B), as indicated. Phosphoproteins were analyzed by SDS–PAGE and autoradiography. Note an unknown phosphoprotein above the eIF2α band (A), which seems to be the consequence of the unspecific copurification of a kinase activity bound to TALON affinity resin. (C) In vitro eIF2α kinase assay of purified GCN2-WT, GCN2-K618R or GCN2-m2, in the absence or presence of SV RNA. Proteins were resolved into 10% SDS–PAGE and transferred to an immobilon-P membrane. The membrane was exposed to autoradiography (upper panels) and then probed with different antisera to detect eIF2α phosphorylated at serine 51 (eIF2α-P), total eIF2α and phosphorylated, and total GCN2 (lower panels) as indicated. (D) Kinase reactions were performed in the presence of the indicated concentrations of SV RNA or GCN2 RNA (as a negative control) and analyzed as described in (A). (E) Phosphorylation of eIF2α by Drosophila PERK, or mammalian PKR, GCN2 and HRI in the absence or presence of either poly(I)–poly(C) (1 μg/ml) or SV RNA (2.5 μg/ml, 0.64 nM). The analysis was carried out as described in (A).
