Figure 4. Protein Mutants Identified and Epistasis Tests.
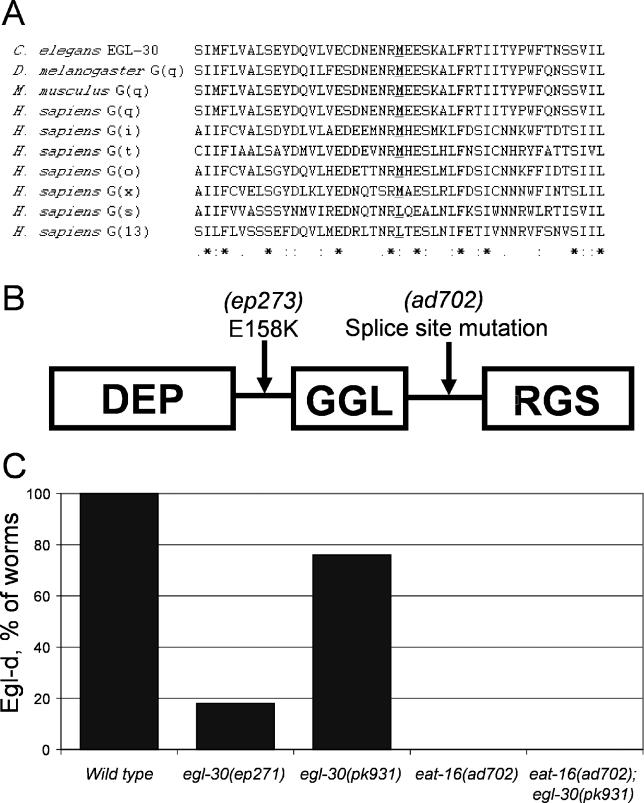
(A) Alignment of a region of EGL-30 containing the M244I amino acid substitution with other G-proteins. The conserved methionine residue that is mutated to isoleucine in the egl-30(ep271) allele is indicated in bold text and underlined.
(B) Diagram showing the domain structure of the EAT-16 protein with relative locations of the E158K substitution found in the eat-16(ep273) allele, and the splice site mutation found in eat16(ad702) strains.
(C) Genetic interactions between mutations in G-αq and RGS. The G-αq/egl-30(ep271) and RGS/eat-16(ad702) mutations confer resistance to BMS-192364. The sensitivity of a G-αq/egl-30(pk931) strain to BMS-192364 is abrogated in the presence of the eat-16(ad702) mutation.