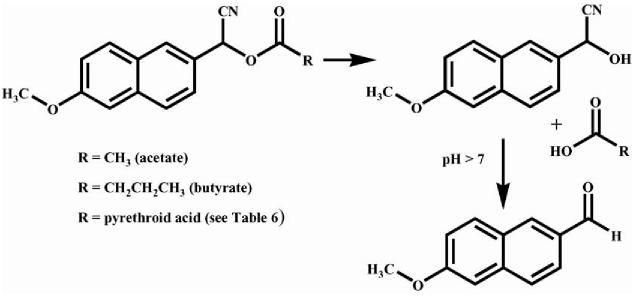
Fig. 2.
Hydrolysis mechanism for the fluorescent carboxylesterase substrates used in this study. The substrates α-cyano(6-methoxy-2-naphthyl)methyl acetate (acetate) and α-cyano(6-methoxy-2-naphthyl)methyl butyrate (butyrate) are shown here, while the remaining pyrethroid surrogates are shown in Table 6. The α-cyano ester is hydrolyzed to produce the corresponding acid and the cyanohydrin, which spontaneously rearranges to the fluorescent aldehyde at neutral and basic pH. The other carboxylesterase substrate used in this study was p-nitrophenyl acetate (PNPA), which has an acetate moiety coupled to p-nitrophenol and produces the yellow p-nitrophenolate anion and acetic acid upon hydrolysis.