Table 6.
Hydrolysis activity of synthetic pyrethroid surrogate substrates
| Compounda | Substrate | Esterase activityb |
|---|---|---|
| 1 | 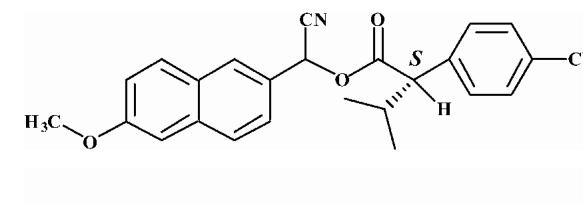 |
8.46 ± 0.09 |
| 2 | 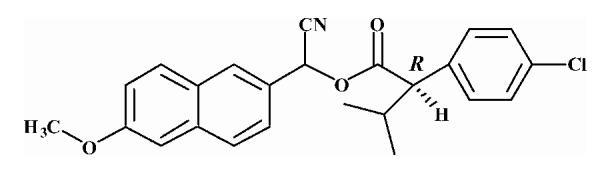 |
20.78 ± 0.68 |
| 3 | 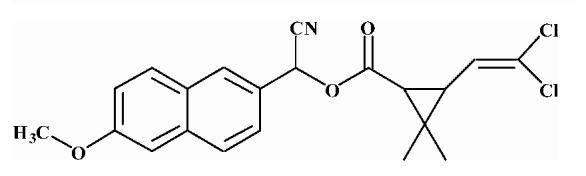 |
37.72 ± 0.70 |
| 4 | 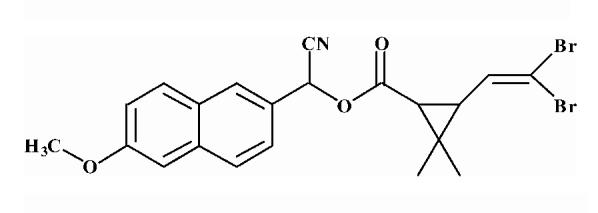 |
5.03 ± 0.26 |
| 5 | 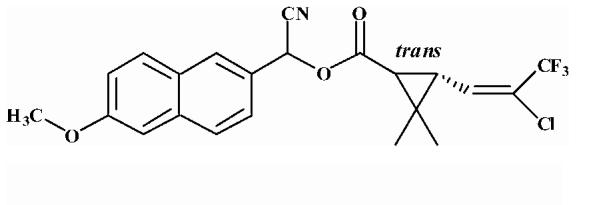 |
14.69 ± 0.43 |
| 6 | 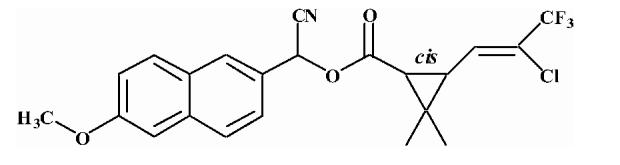 |
3.78 ± 0.10 |
| 7 | 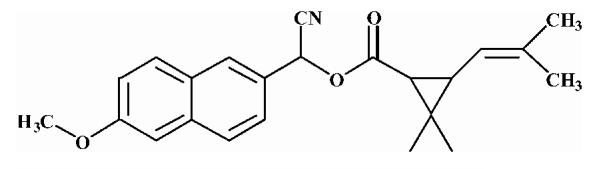 |
38.16 ± 0.84 |
| 8 | 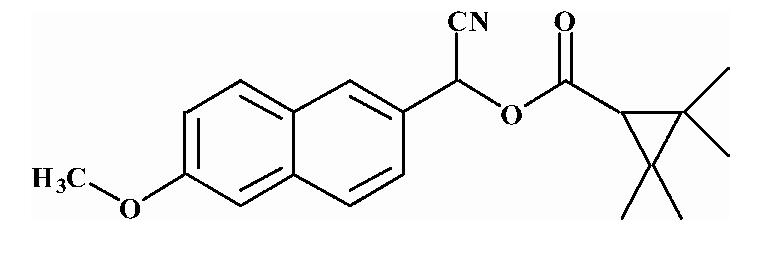 |
6.50 ± 0.28 |
Substrates are pyrethroid surrogates: compound 1 for esfenvalerate, 2 for fenvalerate, 3 for cypermethrin, 4 for deltamethrin, 5 for trans λ-cyhalothrin, 6 for cis λ-cyhalothrin, 7 for phenothrin, and 8 for fenpropathrin.
Activity is in nmol/min/mg protein using a commercial porcine esterase. No significant hydrolysis activity of the substrates (1-8) could be detected with any of the fish tissues examined in this study. Data are the average of 3 independent determinations ± the standard deviation.
