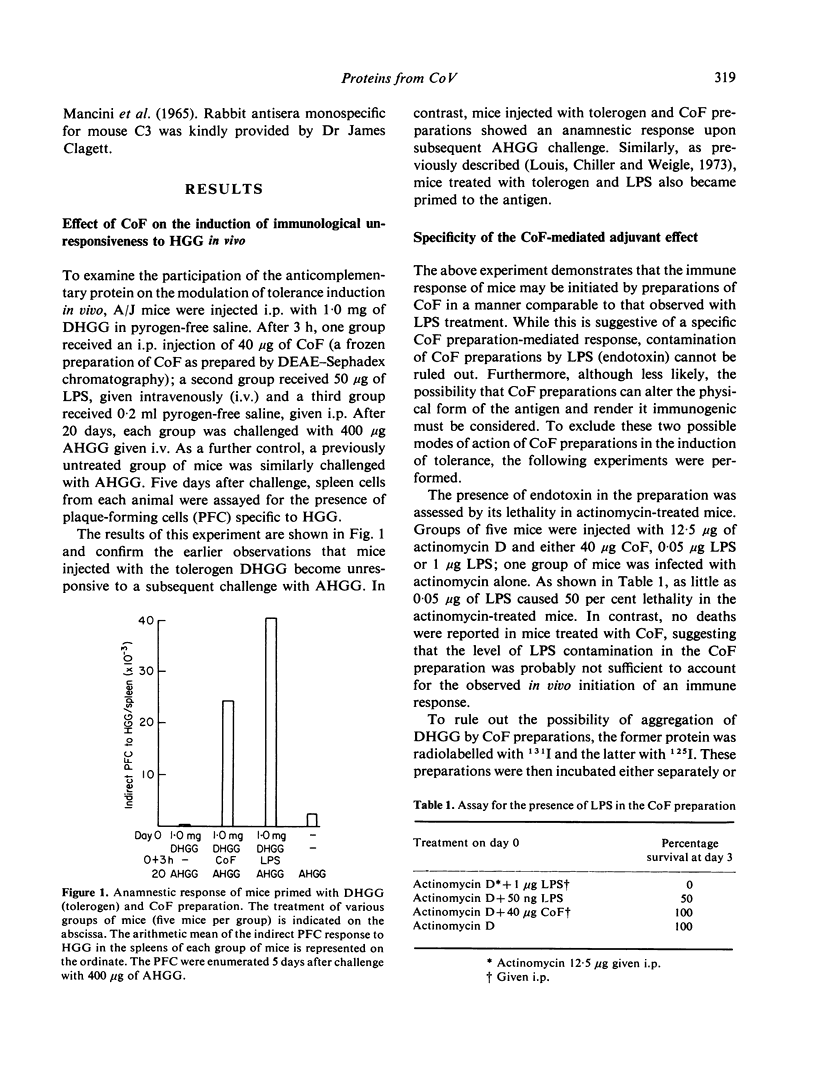
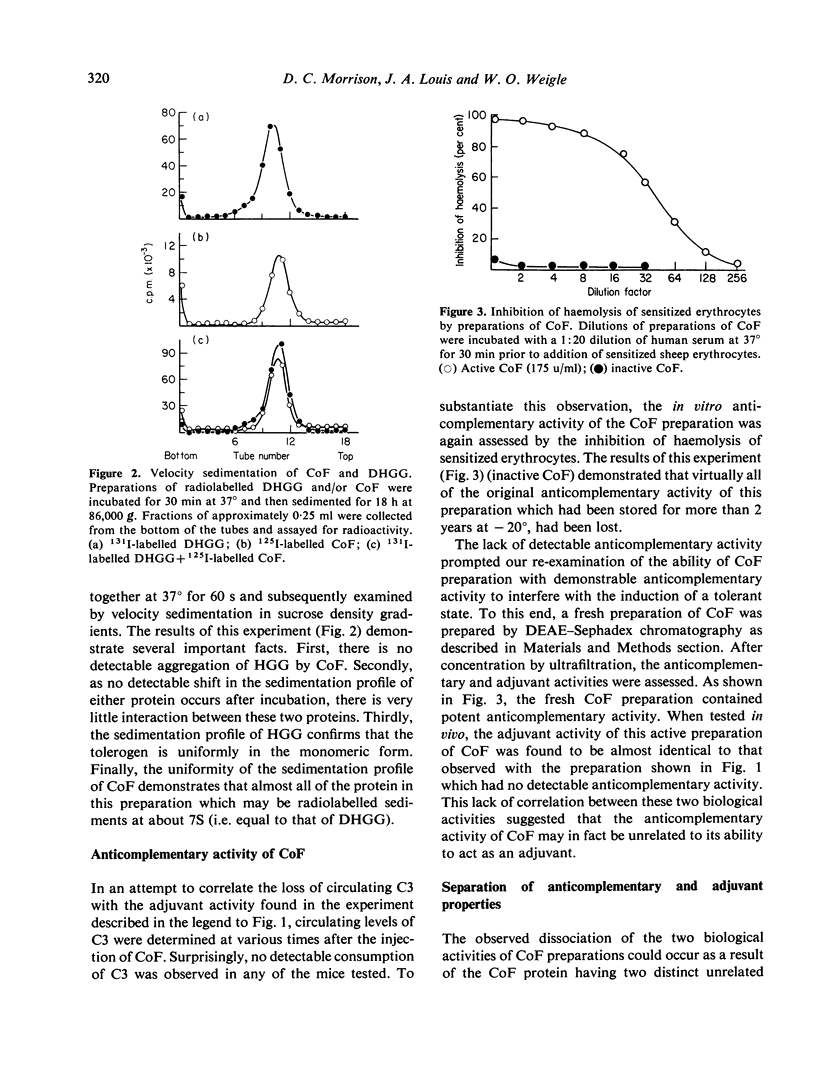
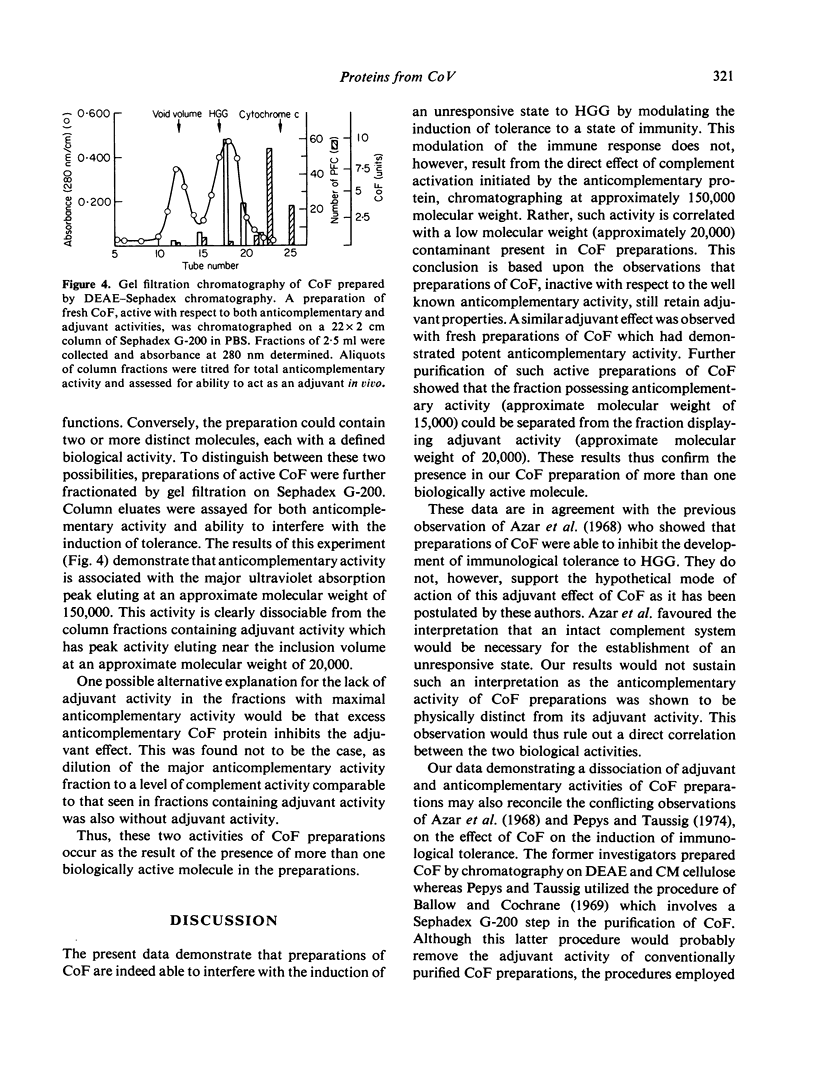
Abstract
The ability of preparations of cobra factor (CoF) to convert C3 via the properdin pathway (anticomplementary activity) has been demonstrated to be unrelated to its ascribed ability to convert a tolerogenic signal into an immunogenic stimulus (adjuvant activity). It has been shown that CoF preparations which have become inactive with regard to anticomplementary activity still retain full adjuvant properties. Further, when both activities are present in preparations of CoF, they can be separated by gel filtration chromatography into a fraction with approximate molecular weight 150,000 which contains anticomplementary activity and a fraction with approximate molecular weight 20,000, which contains adjuvant properties.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azar M. M., Yunis E. J., Pickering P. J., Good R. A. On the nature of immunological tolerance. Lancet. 1968 Jun 15;1(7555):1279–1281. doi: 10.1016/s0140-6736(68)92293-9. [DOI] [PubMed] [Google Scholar]
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Ballow M., Day N. K., Good R. A. Effect of cobra venom factor on the local GVH reaction. I. Partial characterization of a cytotoxic factor from cobra venom for rat lymphocytes. J Immunol. 1973 Feb;110(2):354–361. [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Cooper N. R. Formation and function of a complex of the C3 proactivator with a protein from cobra venom. J Exp Med. 1973 Feb 1;137(2):451–460. doi: 10.1084/jem.137.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESSER D. W. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature. 1961 Sep 16;191:1169–1171. doi: 10.1038/1911169a0. [DOI] [PubMed] [Google Scholar]
- Dukor P., Hartmann K. U. Hypothesis. Bound C3 as the second signal for B-cell activation. Cell Immunol. 1973 Jun;7(3):349–356. doi: 10.1016/0008-8749(73)90199-8. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Pepys M. B. Role of C3 in in vitro lymphocyte cooperation. Nature. 1974 May 10;249(453):159–161. doi: 10.1038/249159a0. [DOI] [PubMed] [Google Scholar]
- Golub E. S., Mishell R. I., Weigle W. O., Dutton R. W. A modification of the hemolytic plaque assay for use with protein antigens. J Immunol. 1968 Jan;100(1):133–137. [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Louis J. A., Chiller J. M., Weigle W. O. The ability of bacterial lipopolysaccharide to modulate the induction of unresponsiveness to a state of immunity. Cellular parameters. J Exp Med. 1973 Dec 1;138(6):1481–1495. doi: 10.1084/jem.138.6.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHERSON C. W. REDUCTION OF PSEUDOMONAS AERUGINOSA AND COLIFORM BACTERIA IN MOUSE DRINKING WATER FOLLOWING TREATMENT WITH HYDROCHLORIC ACID OR CHLORINE. Lab Anim Care. 1963 Oct;13:737–744. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Pepys M. B. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974 Jul 1;140(1):126–145. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Taussig M. J. Complement-independence of tolerance induction. Eur J Immunol. 1974 May;4(5):349–352. doi: 10.1002/eji.1830040508. [DOI] [PubMed] [Google Scholar]
- Waldmann H., Lachmann P. J. The failure to show a necessary role for C3 in the in vitro antibody response. Eur J Immunol. 1975 Mar;5(3):185–193. doi: 10.1002/eji.1830050307. [DOI] [PubMed] [Google Scholar]


