Abstract
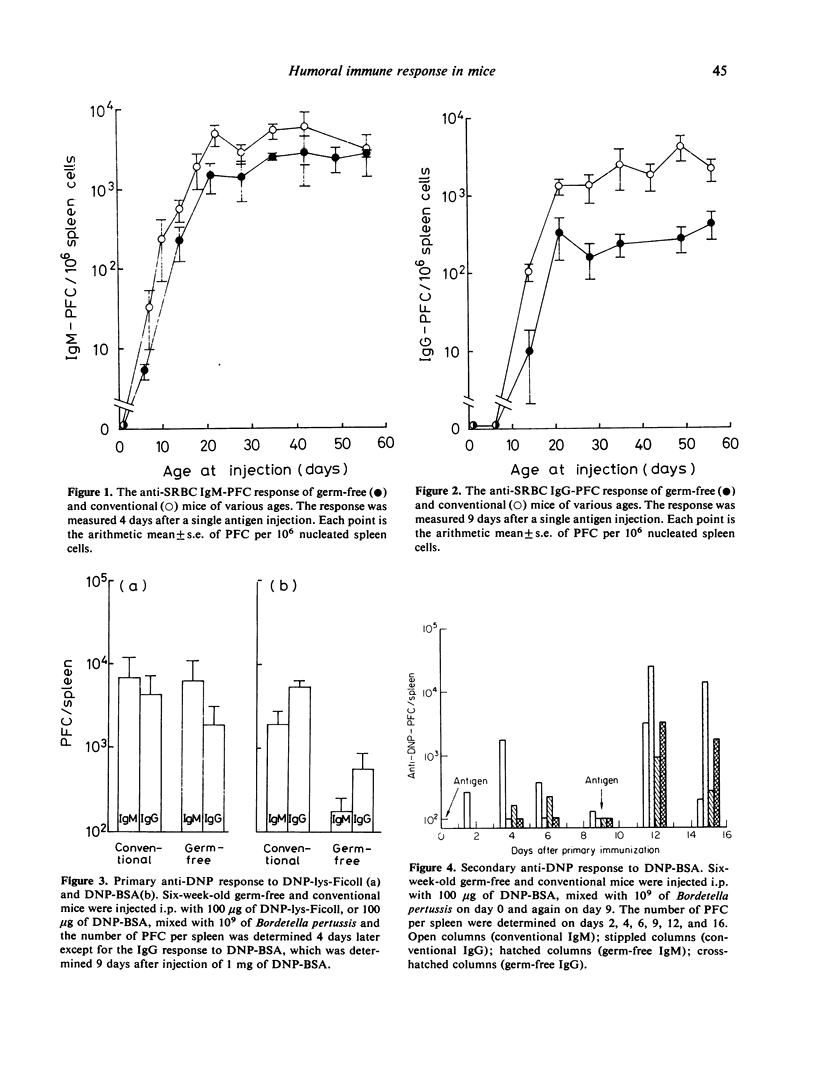
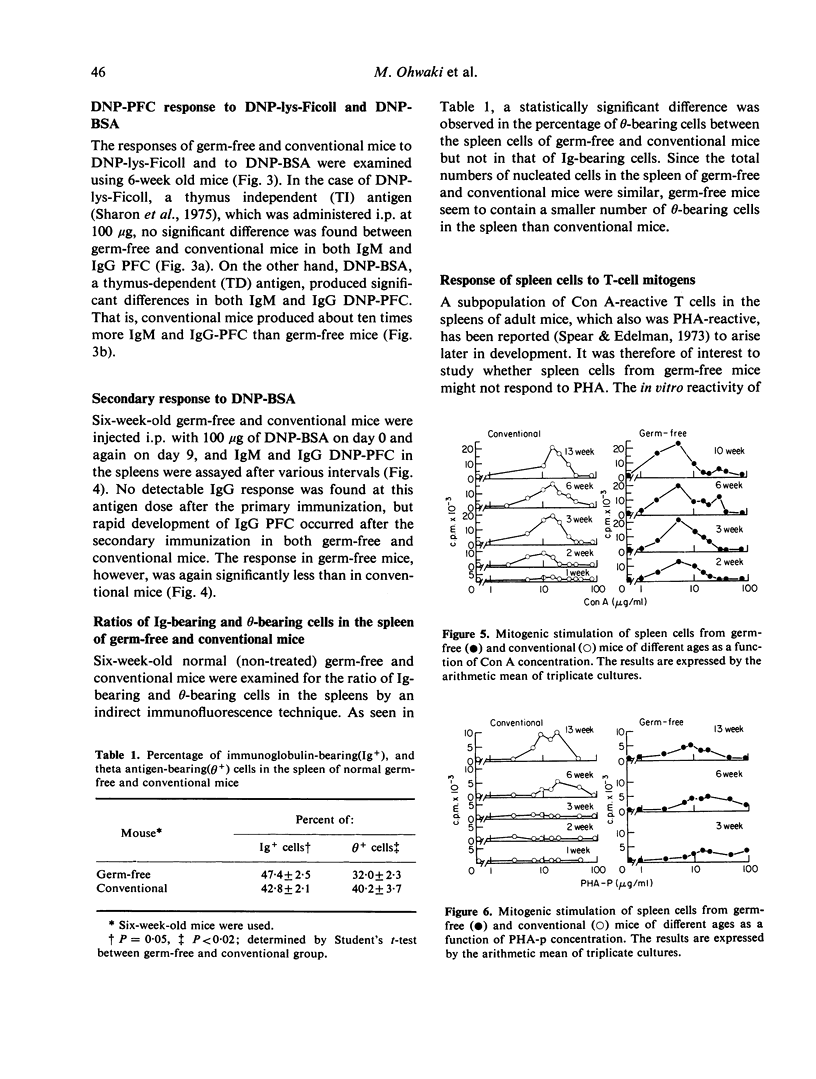
The plaque-forming cell (PFC) responses to sheep red blood cell (SRBC) dinitrophenyl-lysine-Ficoll (DNP-lys-Ficoll), and dinitrophenylated bovine serum albumin (DNP-BSA) have been studied in both germ-free and conventionally reared ICR mice. In germ-free mice, the IgG response to SRBC and the IgM and IgG responses to DNP-BSA were lower than in conventional mice, but no difference was observed in the IgM response to SRBC or the IgM and IgG responses to DNP-lys-Ficoll. Further, the number of 0-bearing cells in the spleen was smaller, and the mitogenic response of spleen cells to PHA was lower in germ-free mice than in conventional mice. These observations suggest that T cells of germ-free mice remain functionally immature.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosma M. J., Makinodan T., Walburg H. E., Jr Development of immunologic competence in germfree and conventional mice. J Immunol. 1967 Aug;99(2):420–430. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Harris G., Pelc S. R., Blackmore D. K. Synthesis of DNA by the spleens of germ-free mice during the primary response to sheep red cells. Eur J Immunol. 1973 Feb;3(2):103–108. doi: 10.1002/eji.1830030210. [DOI] [PubMed] [Google Scholar]
- Hirst J. A., Beverley P. C., Kisielow P., Hoffmann M. K., Oettgen H. F. Ly antigens: markers of T cell function on mouse spleen cells. J Immunol. 1975 Dec;115(6):1555–1557. [PubMed] [Google Scholar]
- Scheid M. P., Hoffmann M. K., Komuro K., Hämmerling U., Abbott J., Boyse E. A., Cohen G. H., Hooper J. A., Schulof R. S., Goldstein A. L. Differentiation of T cells induced by preparations from thymus and by nonthymic agents. J Exp Med. 1973 Oct 1;138(4):1027–1032. doi: 10.1084/jem.138.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert K., Pollard M., Nordin A. Some aspects of humoral immunity in germ-free and conventional SJL-J mice in relation to age and pathology. Cancer Res. 1974 Jul;34(7):1707–1719. [PubMed] [Google Scholar]
- Sharon R., McMaster P. R., Kask A. M., Owens J. D., Paul W. E. DNP-Lys-ficoll: a T-independent antigen which elicits both IgM and IgG anti-DNP antibody-secreting cells. J Immunol. 1975 May;114(5):1585–1589. [PubMed] [Google Scholar]
- Spear P. G., Edelman G. M. Maturation of the humoral immune response in mice. J Exp Med. 1974 Feb 1;139(2):249–263. doi: 10.1084/jem.139.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. B., Wortis H. H. Thymus dependence of antibody response: variation with dose of antigen and class of antibody. Nature. 1968 Nov 30;220(5170):927–928. doi: 10.1038/220927a0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Grundke-Iqbal I., Holmes K. V., Uhr J. W. Cell surface immunoglobulin. VII. Synthesis, shedding, and secretion of immunoglobulin by lymphoid cells of germ-free mice. J Exp Med. 1974 Apr 1;139(4):862–876. doi: 10.1084/jem.139.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostmann B. S., Pleasants J. R., Bealmear P., Kincade P. W. Serum proteins and lymphoid tissues in germ-free mice fed a chemically defined, water soluble, low molecular weight diet. Immunology. 1970 Sep;19(3):443–448. [PMC free article] [PubMed] [Google Scholar]


