Abstract
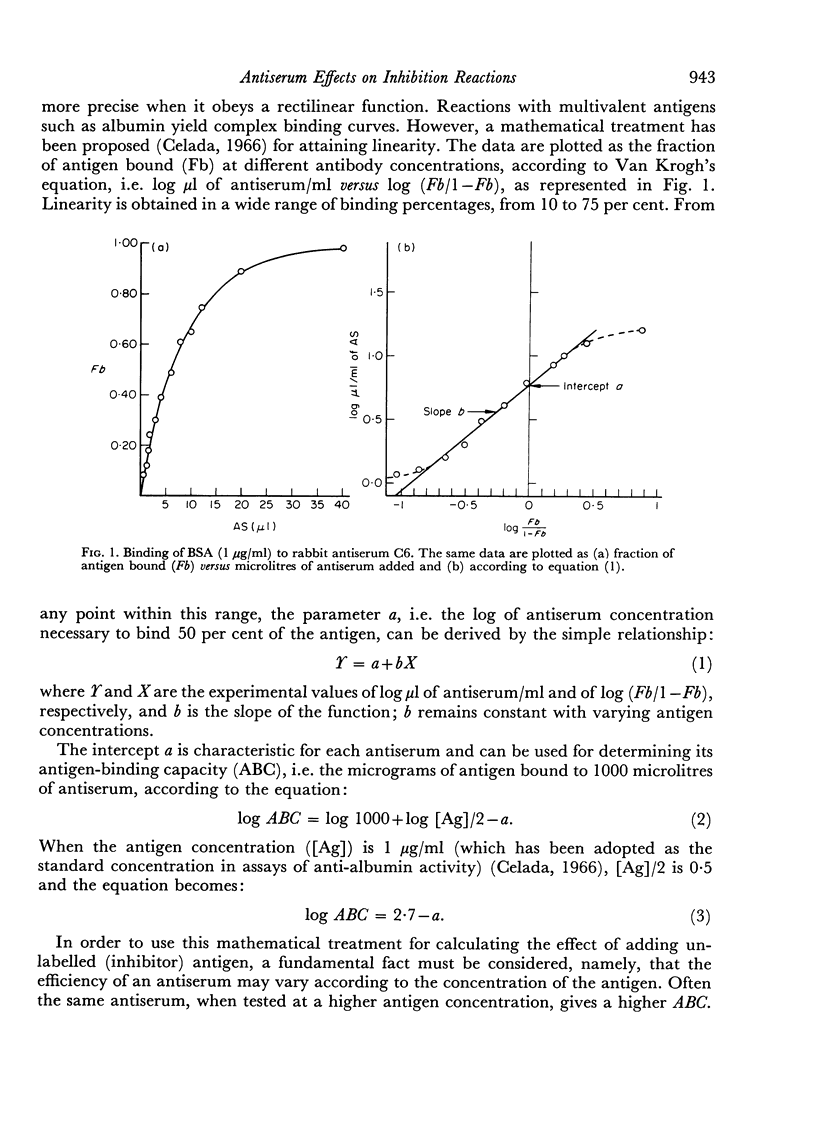
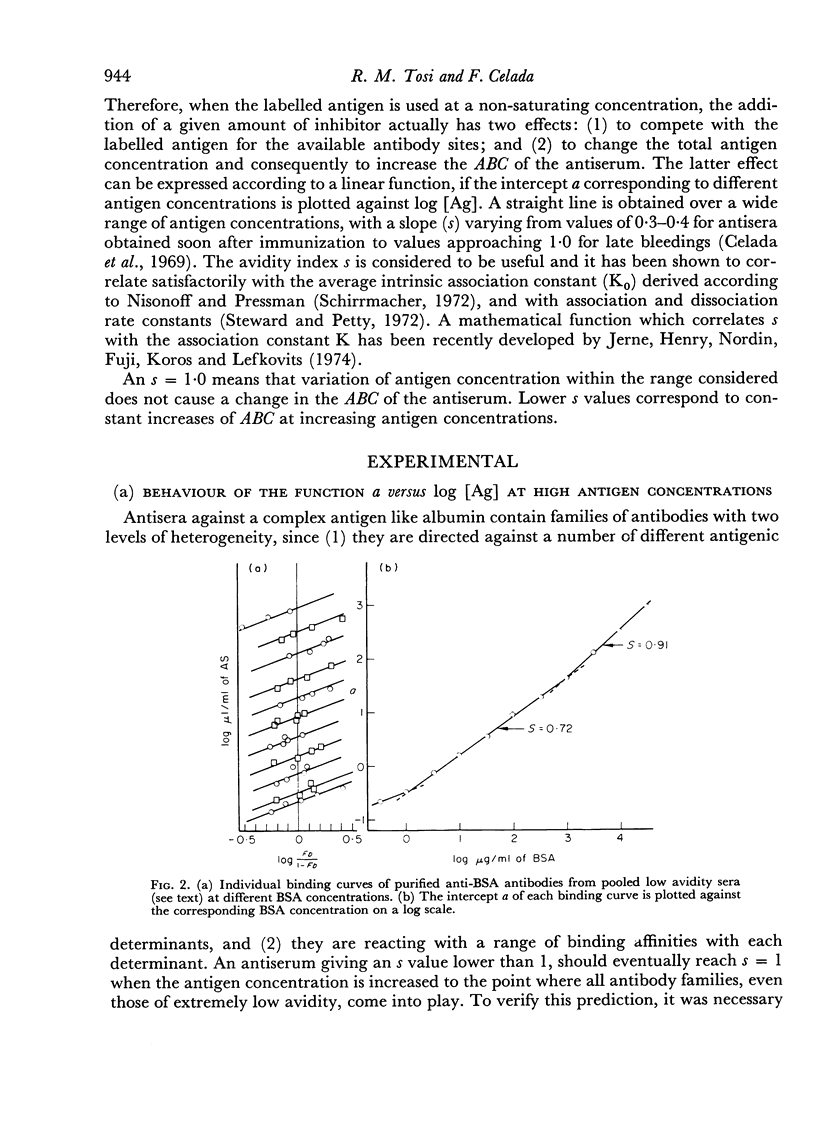
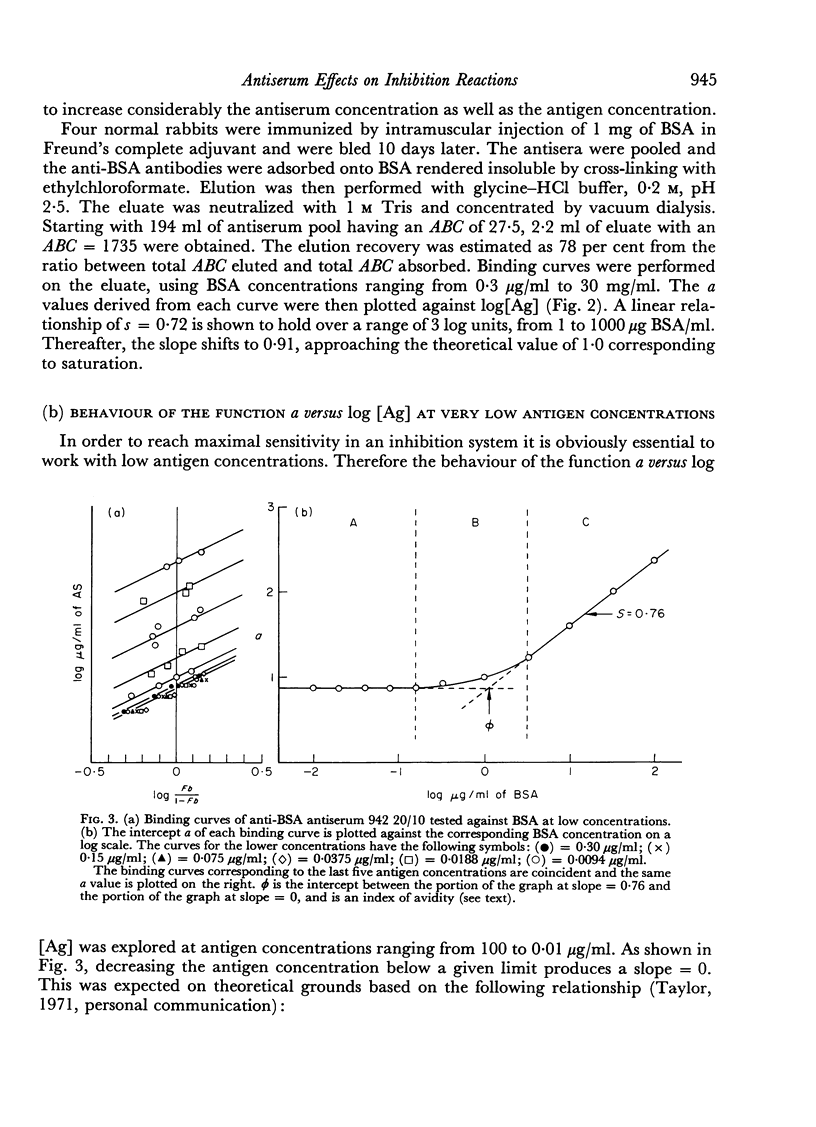
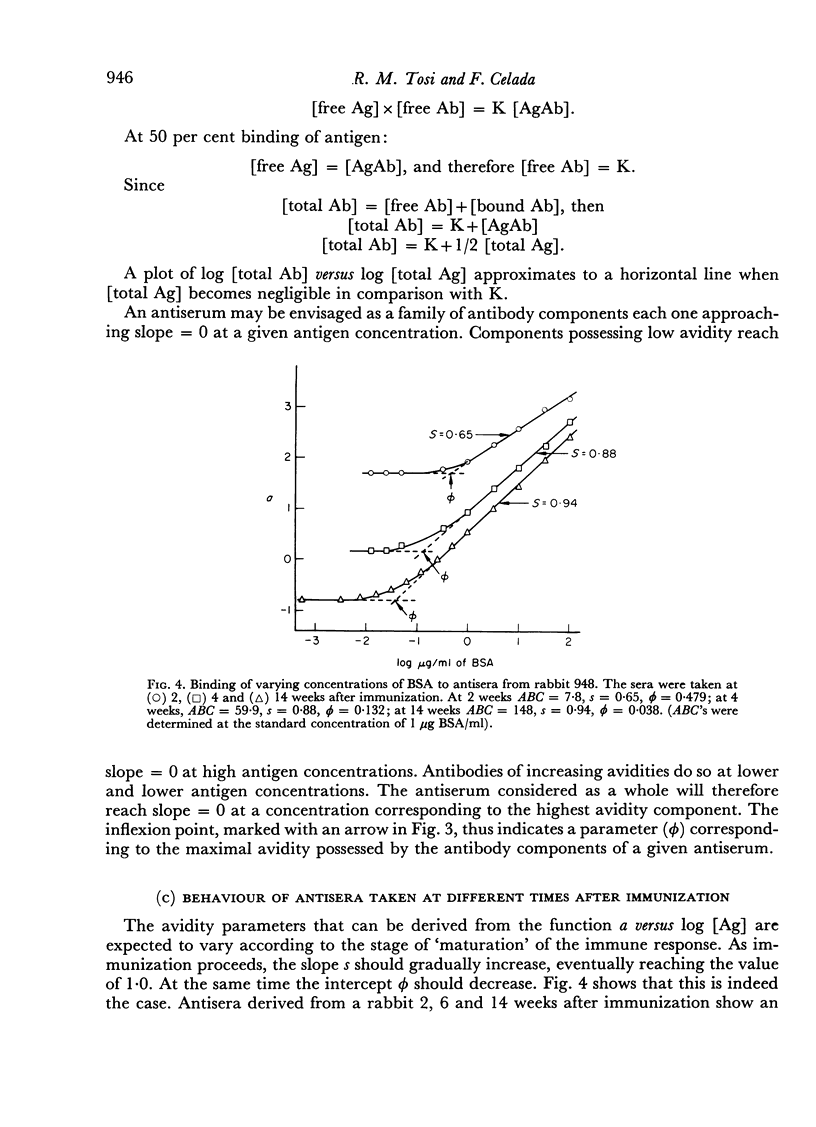
The presence in each antiserum of different antibody components, with different binding affinities, quantitatively affects the outcome of an inhibition reaction. A detailed analysis permits the recognition of the following conditions. (a) At high antigen concentrations, the antiserum is approaching saturation; the only effect of adding unlabelled antigen is competition with the labelled antigen for the available antibody sites. (b) At intermediate antigen concentrations, only a fraction of the antibody components, those of higher affinity, are contributing to the binding reaction; in addition to competition, unlabelled antigen produces an increase in the number of reacting antibody sites. The resultant of these two counteracting effects can be predicted according to the slope of a simple linear function. (c) Lastly, at low antigen concentrations, the added antigen does not have any significant inhibitory effect.
As a result of this analysis, practical indications are available for selecting antisera for radioimmunoassays on the basis of their avidity parameters, for choosing the most suitable antigen range for each antiserum, and for interpreting the shape of inhibition curves in terms of the avidity and heterogeneity of the antiserum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celada F. Quantitative studies of the adoptive immunological memory in mice. I. An age-dependent barrier to syngeneic transplantation. J Exp Med. 1966 Jul 1;124(1):1–14. doi: 10.1084/jem.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada F., Schmidt D., Strom R. Determination of avidity of anti-albumin antibodies in the mouse. Influence of the number of cells transferred on the quality of the secondary adoptive response. Immunology. 1969 Aug;17(2):189–198. [PMC free article] [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givol D., Weinstein Y., Gorecki M., Wilchek M. A general method for the isolation of labelled peptides from affinity-labelled proteins. Biochem Biophys Res Commun. 1970 Feb 20;38(4):825–830. doi: 10.1016/0006-291x(70)90656-x. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., PRESSMAN D. Heterogeneity and average combining constants of antibodies from individual rabbits. J Immunol. 1958 Jun;80(6):417–428. [PubMed] [Google Scholar]
- Schirrmacher V. The synthesis of radioactively labeled sulfanyl-N-chloracetyl tyrosine and its use for determinations of quantities and affinities of anti-p-azobenzene-sulfonate antibodies. Eur J Immunol. 1972 Oct;2(5):430–434. doi: 10.1002/eji.1830020509. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Petty R. E. The antigen-binding characteristics of antibody pools of different relative affinity. Immunology. 1972 Dec;23(6):881–887. [PMC free article] [PubMed] [Google Scholar]
- Tosi S. L., Dubiski S., Mage R. G. Distribution of allotypic specificities A1, A2, A14, and A15 among immunoglobulin G molecules. J Immunol. 1970 Mar;104(3):641–647. [PubMed] [Google Scholar]


