Abstract
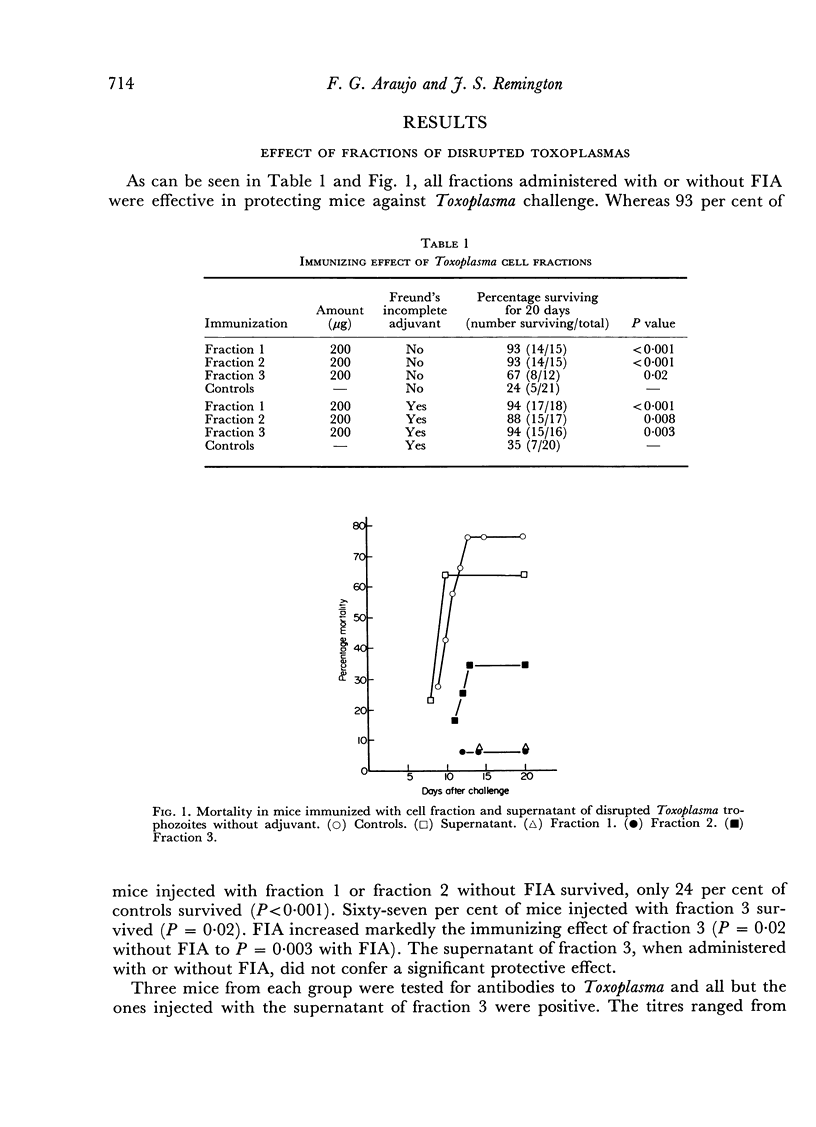
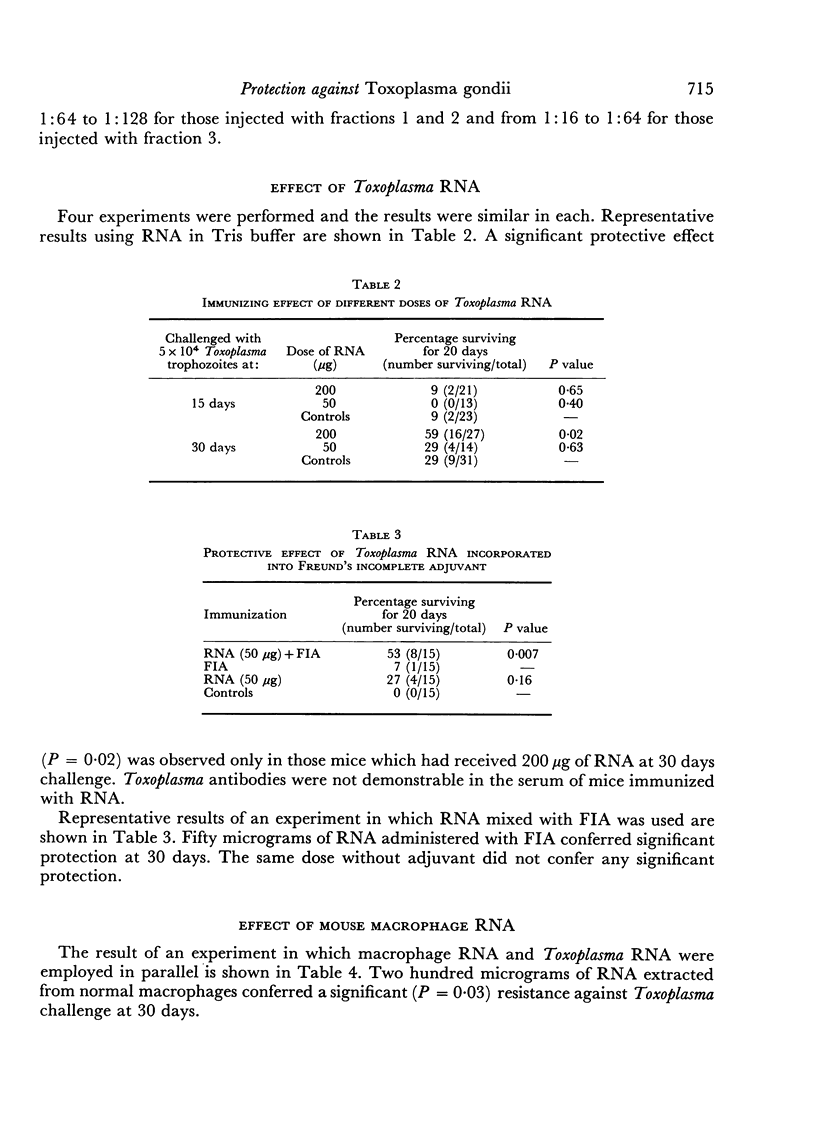
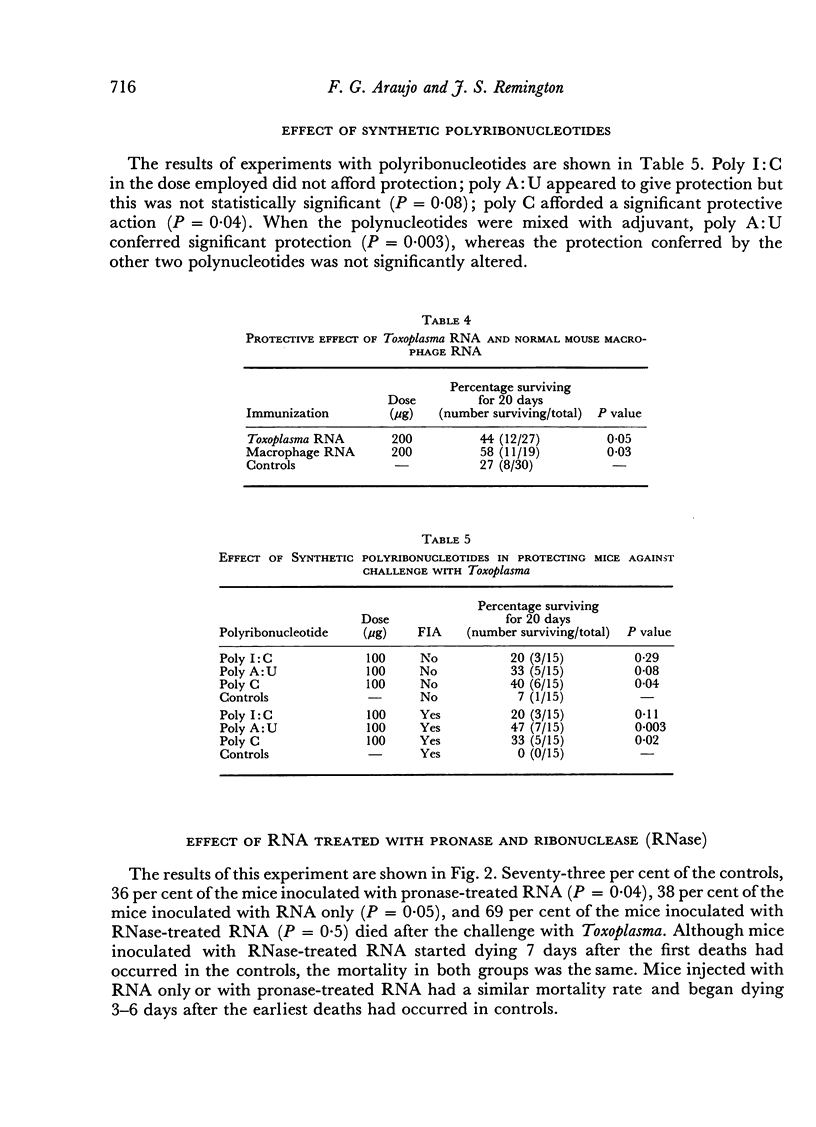
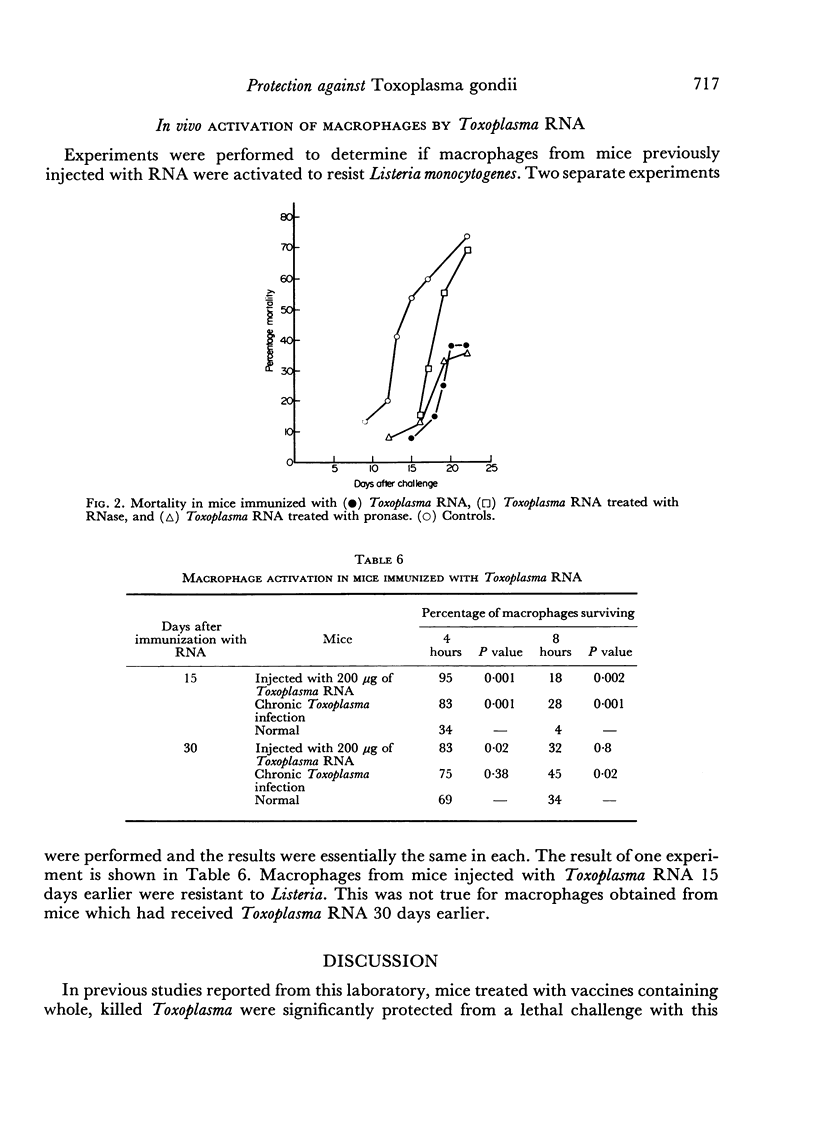
Mice immunized with fractions obtained by centrifugation of disrupted Toxoplasma gondii trophozoites as well as with 200 μg of Toxoplasma ribonucleic acid (RNA) were resistant (as measured by time to death and total mortality) to challenge with Toxoplasma 30 days later. When mice were challenged at 15 days no protection was noted. A dose of 50 μg of Toxoplasma RNA was effective in protecting mice against lethal challenge only when incorporated into Freund's incomplete adjuvant. In studies performed to determine the specificity of the resistance observed, resistance was also noted in mice immunized with 200 μg of RNA extracted from normal mouse peritoneal macrophages, as well as in mice immunized with 100 μg of the synthetic polyribonucleotide polycytidylic acid. Polyadenylicuridylic acid conferred protection only when incorporated into Freund's incomplete adjuvant and polyinosinic—cytidylic acid had no effect. The protection induced by Toxoplasma RNA was eliminated by prior treatment of the preparation with ribonuclease but not by treatment with pronase, suggesting that the moiety responsible for the protective effect was RNA. In experiments designed to explore the mechanism of resistance in the vaccinated mice, macrophages harvested from mice which had been injected with Toxoplasma RNA 15 days earlier were found to be activated in that they resisted challenge with Listeria monocytogenes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., RHODES J. M. IMMUNOGENICITY OF ANTIGEN-CONTAINING RIBONUCLEIC ACID PREPARATIONS FROM MACROPHAGES. Nature. 1965 Jan 30;205:470–474. doi: 10.1038/205470a0. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in mice preimmunized with living or ethyl alcohol-killed vaccines. J Bacteriol. 1969 Feb;97(2):676–683. doi: 10.1128/jb.97.2.676-683.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENKEL J. K., JACOBS L. Ocular toxoplasmosis; pathogenesis, diagnosis and treatment. AMA Arch Ophthalmol. 1958 Feb;59(2):260–279. [PubMed] [Google Scholar]
- Giron D. J., Schmidt J. P., Ball R. J., Pindak F. F. Effect of interferon inducers and interferon on bacterial infections. Antimicrob Agents Chemother. 1972 Jan;1(1):80–81. doi: 10.1128/aac.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R., Baron S. Immunologic-mediated protection of Trypanosoma congolense-infected mice by polyribonucleotides. J Protozool. 1971 Nov;18(4):661–666. doi: 10.1111/j.1550-7408.1971.tb03393.x. [DOI] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. I. Immunogenicity of ribosomal fractions isolated from Salmonella typhimurium and Yersinia pestis. Infect Immun. 1972 Jun;5(6):947–952. doi: 10.1128/iai.5.6.947-952.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Ruskin J., Remington J. S. The use of killed vaccines in immunization against an intracellular parasite: Toxoplasma gondii. J Immunol. 1972 Feb;108(2):425–431. [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Bloomfield M. M., Russell E., Jr, Robinson W. S. The RNA of toxoplasma gondii. Proc Soc Exp Biol Med. 1970 Feb;133(2):623–626. doi: 10.3181/00379727-133-34531. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Synthetic polyanions protect mice against intracellular bacterial infection. Nature. 1970 Apr 25;226(5243):361–363. doi: 10.1038/226361a0. [DOI] [PubMed] [Google Scholar]
- Ruskin J., Remington J. S. Resistance to intracellular infection in mice immunized with Toxoplasma vaccine and adjuvant. J Reticuloendothel Soc. 1971 May;9(5):465–479. [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Detection of delayed hypersensitivity in mice injected with ribonucleic acid-protein fractions of Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):384–389. doi: 10.1128/iai.6.3.384-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Weiss E. Response of mice to injection of ribosomal fraction from group B Neisseria meningitidis. Infect Immun. 1972 Sep;6(3):355–363. doi: 10.1128/iai.6.3.355-363.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Experimental salmonellosis: differential passive transfer of immunity with serum and cells obtained from ribosomal and ribonucleic acid-immunized mice. J Reticuloendothel Soc. 1971 May;9(5):491–502. [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J., Berry L. J. Immunogenicity of Ribonucleic Acid Preparations Obtained from Salmonella typhimurium. Infect Immun. 1970 Jun;1(6):574–582. doi: 10.1128/iai.1.6.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J. Isolation and partial characterization of an immunogenic moiety obtained from Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):140–148. doi: 10.1128/jb.100.1.140-148.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. J., Waitz J. A., Came P. E. Induction of resistance to bacterial infections of mice with poly I-poly C. Nature. 1970 Apr 11;226(5241):170–170. doi: 10.1038/226170a0. [DOI] [PubMed] [Google Scholar]
- Winston S. H., Berry L. J. Antibacterial immunity induced by ribosomal vaccines. J Reticuloendothel Soc. 1970 Jul;8(1):13–24. [PubMed] [Google Scholar]
- Winston S., Berry L. J. Immunity induced by ribosomal extracts from Staphylococcus aureus. J Reticuloendothel Soc. 1970 Jul;8(1):66–73. [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Factors affecting immunogenic activity of mycobacterial ribosomal and ribonucleic acid preparations. J Bacteriol. 1969 Jul;99(1):42–50. doi: 10.1128/jb.99.1.42-50.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Failure of synthetic polynucleotides to affect the immunogenicity of mycobacterial ribonucleic Acid and ribosomal protein preparations. Infect Immun. 1971 Jan;3(1):149–153. doi: 10.1128/iai.3.1.149-153.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Immunogenic mycobacterial ribosomal and ribonucleic Acid preparations: chemical and physical characteristics. Infect Immun. 1970 Nov;2(5):659–668. doi: 10.1128/iai.2.5.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


