Abstract
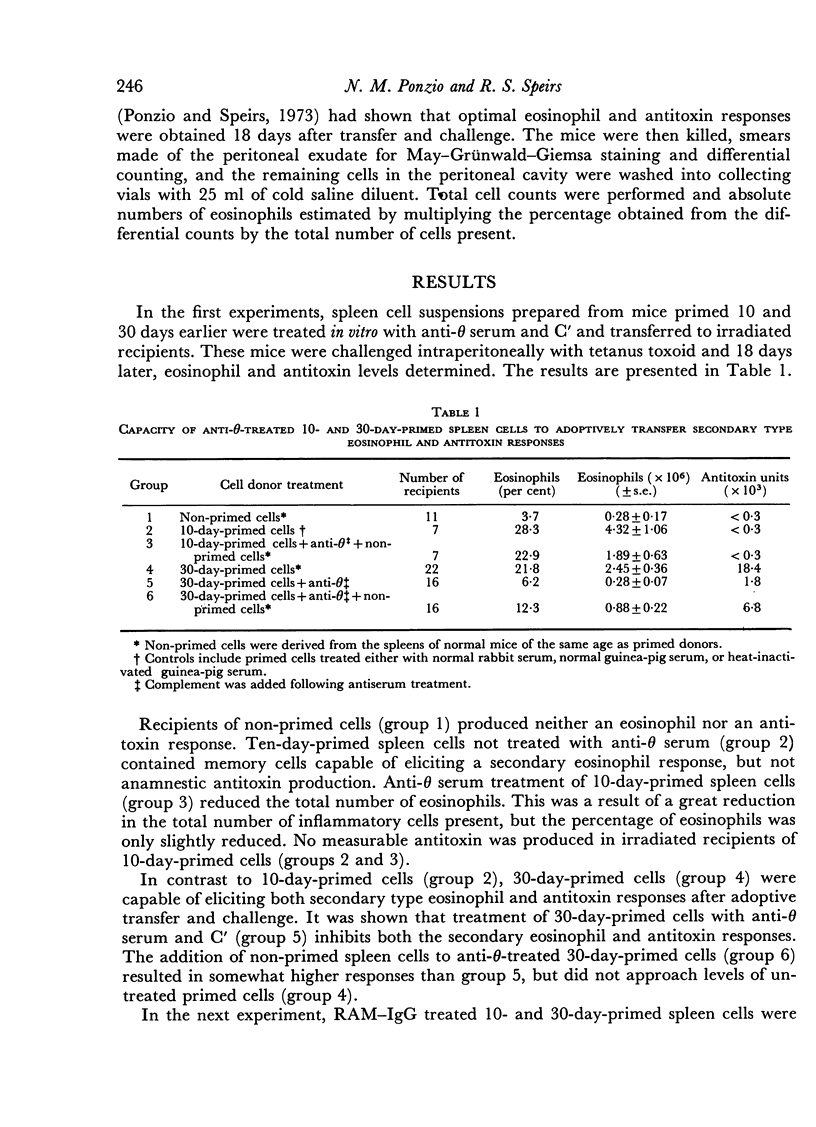
Spleens from mice primed with tetanus toxoid 30 days earlier contain memory cells capable of adoptively transferring secondary type cell-mediated (eosinophil) and humoral (antitoxin) responses to irradiated, reconstituted recipients. Spleen cells derived from 10-day-primed donors, on the other hand, possess the capacity after transfer to elicit secondary type eosinophil responses, but not anamnestic antitoxin responses. Treatment of 30-day-primed cells with anti-theta serum and C' prevented transfer of memory for both responses, whereas similar treatment with rabbit anti-mouse IgG (RAM-IgG) serum and C' only inhibited transfer of memory for the antitoxin response. Addition of non-primed spleen cells to antisera-treated primed cells failed to restore secondary type responses. Recombination of 30-day-primed anti-theta and RAM-IgG-treated cells re-established the capacity to transfer these responses. To determine whether the same T cells which mediate the eosiniphil response also act as helper cells in antitoxin production, antisera treated 10- and 30-day-primed cells were combined prior to transfer. Ten-day-primed T cells induced eisoniphil responses and also co-operated with 30-day-primed B cells to produce antitoxin. In contrast, 30-day-primed T cells elicited eisinophil responses, but were unable to induce antitoxin production when combined with anti-theta-treated 10-day-primed cells. These results indicate that B memory cells are not present in the spleens of the donor mice 10 days after priming, but T memory cells are present. It is concluded that primed T cells mediated both eosinophil and antitoxin responses, while B memory cells are involved only with antitoxin production. Following subcutaneous priming T memory cells are present in the spleen prior to B memory cells, and T memory cells which mediate the eosinophil response at 10 days after priming also augment the production of antitoxin by B memory cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basten A., Boyer M. H., Beeson P. B. Mechanism of eosinophilia. I. Factors affecting the eosinophil response of rats to Trichinella spiralis. J Exp Med. 1970 Jun 1;131(6):1271–1287. doi: 10.1084/jem.131.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byfield P., Salerno A. Relative resistance of early memory cells in mice primed with sheep erythrocytes to cytotoxic effects of anti-theta serum. Cell Immunol. 1973 May;7(2):328–331. doi: 10.1016/0008-8749(73)90255-4. [DOI] [PubMed] [Google Scholar]
- Cantor H. The effects of anti-theta antiserum upon graft-versus-host activity of spleen and lymph node cells. Cell Immunol. 1972 Mar;3(3):461–469. doi: 10.1016/0008-8749(72)90251-1. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature. 1970 Dec 26;228(5278):1308–1309. doi: 10.1038/2281308a0. [DOI] [PubMed] [Google Scholar]
- Chapuis B., Brunner K. T. Cell mediated immune reactions "in vitro". Reactivity of lymphocytes from animals sensitized to chicken erythrocytes, tuberculin or transplantation antigens. Int Arch Allergy Appl Immunol. 1971;40(3):321–339. [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H., Benacerraf B. Hapten-specific IgE antibody responses in mice. II. Cooperative interactions between adoptively transferred T and B lymphocytes in the development of IgE response. J Exp Med. 1973 Sep 1;138(3):538–556. doi: 10.1084/jem.138.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPSEN J., Jr Bio-assay of four tetanus toxoids (aluminum precipitated) in mice, guinea pigs and humans. J Immunol. 1953 Apr;70(4):426–434. [PubMed] [Google Scholar]
- Kay A. B., Austen K. F. The IgE-mediated release of an eosinophil leukocyte chemotactic factor from human lung. J Immunol. 1971 Sep;107(3):899–902. [PubMed] [Google Scholar]
- Kettman J. Delayed hypersensitivity: is the same population of thymus-derived cells responsible for cellular immunity reactions and the carrier effect? Immunol Commun. 1972;1(3):289–299. doi: 10.3109/08820137209022942. [DOI] [PubMed] [Google Scholar]
- McGarry M. P., Speirs R. S., Jenkins V. K., Trentin J. J. Lymphoid cell dependence of eosinophil response to antigen. J Exp Med. 1971 Sep 1;134(3 Pt 1):801–814. doi: 10.1084/jem.134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Sprent J. Cell-to-cell interaction in the immune response. VI. Contribution of thymus-derived cells and antibody-forming cell precursors to immunological memory. J Exp Med. 1971 Jul 1;134(1):66–82. doi: 10.1084/jem.134.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Takahashi T., Thorbecke G. J. Surface antigens of immunocompetent cells. 3. In vitro studies of the role of B and T cells in immunological memory. J Exp Med. 1972 Oct 1;136(4):663–675. doi: 10.1084/jem.136.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish W. E. Eosinophilia. I. Eosinophilia in guinea-pigs mediated by passive anaphylaxis and by antigen-antibody complexes containing homologous IgG1a and IgG1b. Immunology. 1972 Jun;22(6):1087–1098. [PMC free article] [PubMed] [Google Scholar]
- Parish W. E. Investigations on eosinophilia. The influence of histamine, antigen-antibody complexes containing gamma-1 or gamma-2 globulins, foreign bodies (phagocytosis) and disrupted mast cells. Br J Dermatol. 1970 Jan;82(1):42–64. doi: 10.1111/j.1365-2133.1970.tb02193.x. [DOI] [PubMed] [Google Scholar]
- Piazzi S. E. Metodo di preparazione ed analisi delle gamma-globuline antitetaniche purificate dal siero di bovini iperimmunizzati. Prog Immunobiol Stand. 1970;4:114–120. [PubMed] [Google Scholar]
- Ponzio N. M., Speirs R. S. Lymphoid cell dependence of eosinophil response to antigen. 3. Comparison of the rate of appearance of two types of memory cells in various lymphoid tissues at different times after priming. J Immunol. 1973 May;110(5):1363–1370. [PubMed] [Google Scholar]
- Ponzio N. M., Speirs R. S. Lymphoid cell dependence of eosinophil response to antigen. IV. Effects of in vitro x-irradiation on adoptive transfer of anamnestic cellular and humoral responses. Proc Soc Exp Biol Med. 1974 Apr;145(4):1178–1180. doi: 10.3181/00379727-145-37976. [DOI] [PubMed] [Google Scholar]
- Ponzio N. M., Speirs R. S. Lymphoid cell dependence of eosinophil response to antigen. V. Evidence for the induction of separate memory cells mediating cellular and humoral responses to tetanus toxoid. Z Immunitatsforsch Exp Klin Immunol. 1974 Apr;146(5):405–413. [PubMed] [Google Scholar]
- Raff M. C. T and B lymphocytes in mice studied by using antisera against surface antigenic markers. Am J Pathol. 1971 Nov;65(2):467–478. [PMC free article] [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse thymic iso-antigens. Nature. 1966 Jan 29;209(5022):521–523. doi: 10.1038/209521b0. [DOI] [PubMed] [Google Scholar]
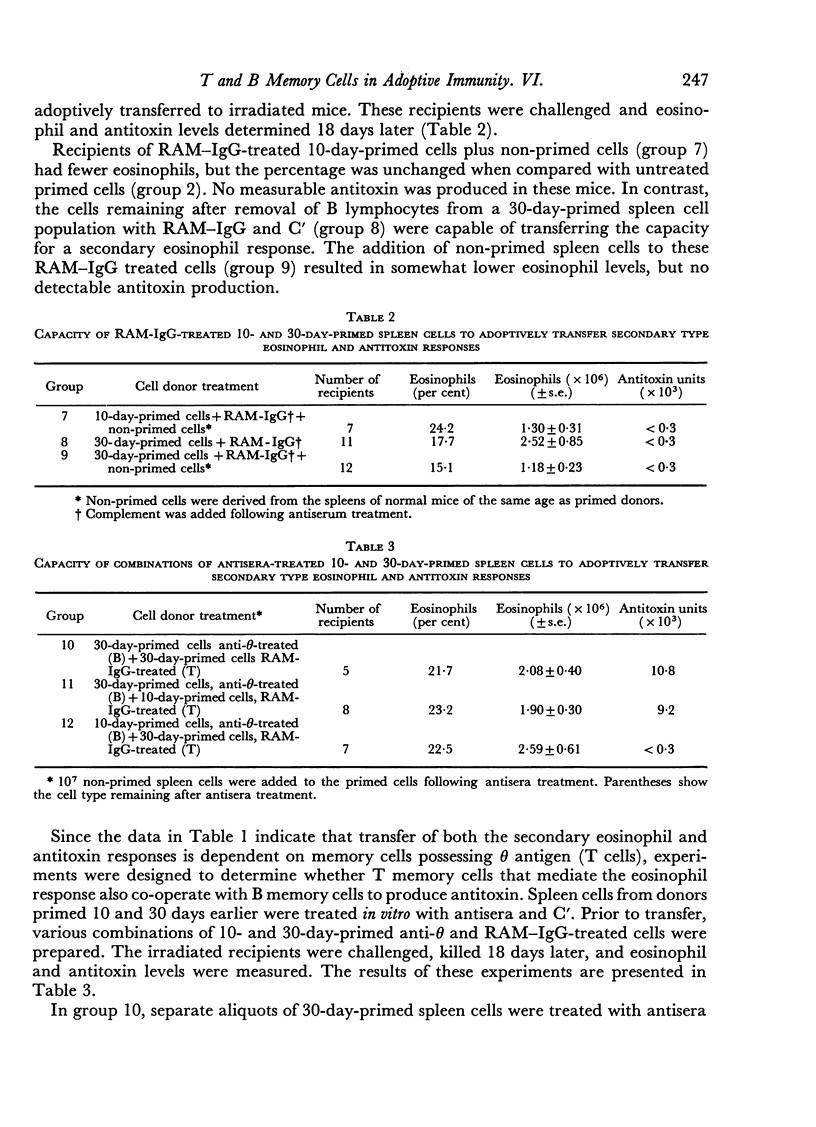
- SPEIRS R. S., WENCK U. Eosinophil response to toxoids in actively and passively immunized mice. Proc Soc Exp Biol Med. 1955 Dec;90(3):571–574. doi: 10.3181/00379727-90-22101. [DOI] [PubMed] [Google Scholar]
- Schimpl A., Wecker E. Inhibition of in vitro immune response by treatment of spleen cell suspensions with anti-theta serum. Nature. 1970 Jun 27;226(5252):1258–1259. doi: 10.1038/2261258a0. [DOI] [PubMed] [Google Scholar]
- Speirs R. S., Gallagher M. T., Rauchwerger J., Heim L. R., Trentin J. J. Lymphoid cell dependence of eosinophil response to antigen. II. Location of memory cells and their dependence upon thymic influence. Exp Hematol. 1973;1(3):150–158. [PubMed] [Google Scholar]
- Speirs R. S., Turner M. X. The eosinophil response to toxoids and its inhibition by antitoxin. Blood. 1969 Sep;34(3):320–330. [PubMed] [Google Scholar]
- Takahashi T., Carswell E. A., Thorbecke G. J. Surface antigens of immunocompetent cells. I. Effect of theta and PC.1 alloantisera on the ability of spleen cells to transfer immune responses. J Exp Med. 1970 Dec 1;132(6):1181–1190. doi: 10.1084/jem.132.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Mond J. J., Carswell E. A., Thorbecke G. J. The importance of theta and Ig hearing cells in the immune response to various antigens. J Immunol. 1971 Dec;107(6):1520–1526. [PubMed] [Google Scholar]
- Vischer T. L. Immunoglobulin-like surface molecules and theta antigen during the specific and non-specific stimulation of mouse spleen cells in vitro. Clin Exp Immunol. 1972 Aug;11(4):523–534. [PMC free article] [PubMed] [Google Scholar]
- Vischer T. L., Jaquet C. Effect of antibodies against immunoglobulins and the theta antigen on the specific and non-specific stimulation of mouse spleen cells in vitro. Immunology. 1972 Feb;22(2):259–266. [PMC free article] [PubMed] [Google Scholar]


