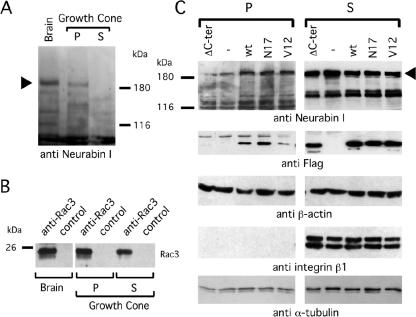
Figure 5.
Rac3 and Neurabin I are detectable in growth cones of rat brain and Neurabin I accumulates to the cytoskeleton upon FlagRac3 expression. (A) Growth cones were purified from embryonic rat brains and fractionated in Triton X-100-soluble cytosolic-enriched fraction (S) and Triton X-100-insoluble pellet (P), which is enriched of cytoskeletal proteins. Anti-Neurabin I immunoblot shows the 180-kDa band of Neurabin I (arrowhead) in total brain extract and in the P fraction of the growth cones. (B) Anti-Rac3 or control immunoprecipitations of the aforementioned extracts were resolved on SDS-PAGE, and endogenous Rac3 was revealed by anti-Rac3 immunoblot. (C) SK-N-BE cells transiently expressing wild-type or mutated FlagRac3 proteins were lysed. Total lysates were separated in S and P fractions as described above and analyzed in immunoblot with the indicated antibodies (anti-Neurabin I). Note that the expression of FlagRac3V12, FlagRac3N17 and FlagRac3 proteins increases the amount of 180-kDa Neurabin I (arrowhead) in the P fractions compared with untransfected cells. A concomitant reduction of Neurabin I levels in the corresponding S fractions is also evident. No variation is observed in FlagRac3ΔC-ter transfected cells. The levels of the Flagged-recombinant proteins (anti-FLAG) are shown as a control of transfection. Anti-integrin β1 immunoblot reveals the absence of membrane bound proteins in the P fractions, whereas anti-β-actin and anti-α-tubulin immunoblots confirm the presence of cytoskeletal proteins in the Triton X-100-insoluble fractions.