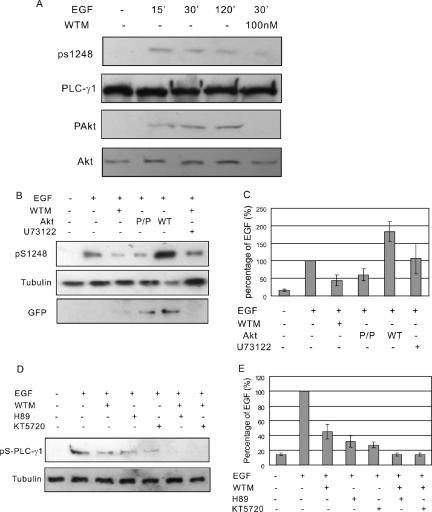
Figure 6.
AKT mediates EGF-induced serine phosphorylation of PLC-γ1. (A) COS-7 cells were treated with 100 ng/ml EGF for indicated times with or without 100 nM wortmannin. The total cell lysates were immunoblotted with antibodies to Akt, pAkt, PLC-γ1, and pS1248 of PLC-γ1. (B) COS-7 cells without transfection were treated with 100 ng/ml EGF or EGF and 100 nM wortmannin or U73122. COS-7 cells transfected with GFP-Akt or GFP-tagged mutant Akt P/P were either not treated or treated with EGF. Then, the cell lysates were immunoblotted with anti-pS1248 antibody. Immunoblotting with anti-tubulin antibody was used as loading control and immunoblotting with anti-GFP antibody was used to show the expression level of transfected Akt. (C) Quantification of the data from three independent experiments as described in B. The PLC-γ1 S1248 phosphorylation levels were expressed as the percentage of the level after EGF stimulation. (D) COS-7 cells were treated with 100 ng/ml EGF or EGF with PKA inhibitors H89/KT5270 or with both wortmannin and H89/KT5270. Then, the cell lysates were immunoblotted with anti-pS1248 antibody. (E) Quantification of the data from three independent experiments as described in D. The PLC-γ1 S1248 phosphorylation levels were expressed as the percentage of the level after EGF stimulation.