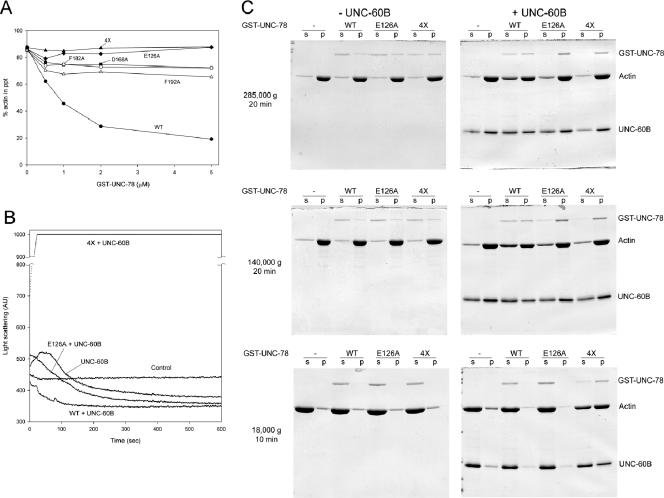
Figure 4.
The 4X UNC-78 mutant is defective in actin filament disassembly. (A) F-actin pelleting assays were performed with 10 μM F-actin, 20 μM UNC-60B, and various concentrations of wild-type or mutant GST-UNC-78 proteins at 285,000 × g for 20 min. Wild-type (black circles) decreased pelletable actin, and D168A (black squares), F182A (white circles), and F192A (white triangles) exhibited reduced activity, whereas E126A (black diamonds) and 4X (black triangles) failed to decrease pelletable actin under these conditions. (B) Light scattering assays were performed with 5 μM F-actin, 5 μM UNC-60B, with or without 1 μM wild type, E126A, or 4X GST-UNC-78. Control experiments were done with F-actin alone. Wild type enhanced actin disassembly and/or severing and rapidly decreased light scattering. E126A exhibited reduced activity and slowly decreased the light scattering signal. In contrast, 4X rapidly increased the signal above the measurable range (arbitrary units [AU] 1000). In the absence of UNC-60B, wild type, E126A, and 4X did not alter light scattering compared with the control (our unpublished data). (C) F-actin pelleting assays were performed with 10 μM F-actin, with or without 10 μM UNC-60B and with or without 1 μM wild-type or mutant GST-UNC-78 proteins at three different centrifugal forces as indicated on the left. The supernatants (s) and pellets (p) were examined by SDS-PAGE. At 285,000 × g for 20 min, only wild type increased actin in the supernatant in the presence of UNC-60B. At 140,000 × g for 20 min, E126A slightly increased actin in the supernatant, but not as much as wild type. In contrast, at 18,000 × g for 10 min, only 4X caused sedimentation of actin in the presence of UNC-60B, suggesting that F-actin bundles were formed.