FIG.9.
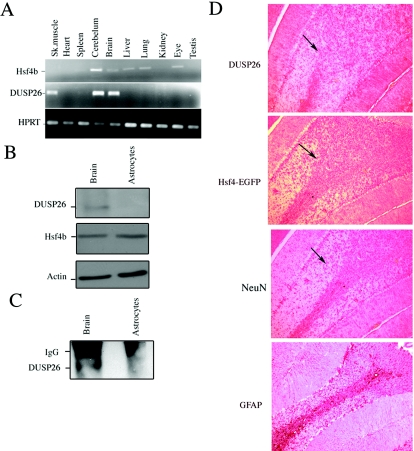
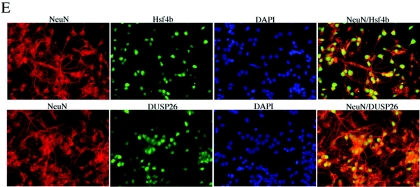
Hsf4b and DUSP26 coexpress in the mouse brain. (A) cDNAs were prepared from the indicated tissues and PCR amplified (20 cycles) using primers specific to Hsf4b, DUSP26, or HPRT. (B) Immunoblotting experiments were performed using 30 μg of cell lysate prepared from whole murine brain or primary astrocyte cultures. The membranes were blotted using antibody to DUSP26, Hsf4b, or β-actin. (C) The same cell lysates from panel B were used to detect interactions between endogenous DUSP26 and Hsf4b. Cell lysates were subjected to immunoprecipitation using antibody to Hsf4b and blotted using antibody to DUSP26. These experiments were performed three times, and results were consistent. IgG, immunoglobulin G. (D) Immunohistochemical staining of paraffin-embedded sections of the cerebellum showing the endogenous expression of DUSP26, Hsf4-EGFP (targeted knock-in mice) (33), glial fibrillary acidic protein (GFAP) (astrocyte specific), and NeuN (neuron specific) using appropriate antibodies. Arrows indicate the areas where Hsf4b, DUSP26, and NeuN potentially coexpress. (E) Rat primary cortical neuronal cultures from postnatal day 0 were grown for 3 days; cells were then fixed with 4% paraformaldehyde and were immunostained using antibody to NeuN and Hsf4b or NeuN and DUSP26. Nuclei were stained using 4′,6′-diamidino-2-phenylindole (DAPI). Coimmunostaining of both Hsf4b and DUSP26 was not performed because antibodies to both Hsf4b and DUSP26 were rabbit polyclonal. However, almost all of the NeuN-positive cells were immunostained with both Hsf4b and DUSP26 (right panels).