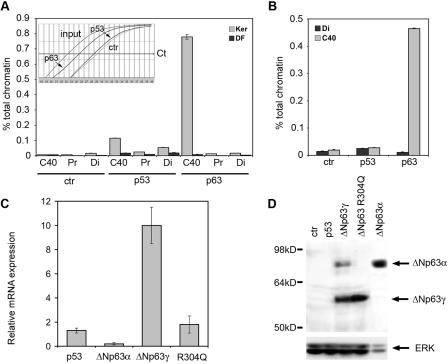
FIG. 6.
p63 binds to the C40 enhancer in the chromatin context and can induce its own transcription. (A) p63 specifically associates with C40 in keratinocyte nuclei. Chromatin extracted from cross-linked mouse primary keratinocytes (Ker) or dermal fibroblasts (DF) was immunoprecipitated in parallel using anti-p63, anti-p53, or anti-ERK (ctr) antibodies. Precipitated DNA samples were used as a template for real-time PCR with oligonucleotide primers recognizing the C40 enhancer, a flanking genomic region 1.8 kb from C40 (Pr), and a distal genomic region (Di: C44 to C45 conserved region). The amount of precipitated DNA was calculated relative to the total input chromatin and expressed as percentage of the total according to the following formula: % total = 2ΔCt × 5, where ΔCt = Ct (input) − Ct (immunoprecipitation), and Ct is cycle threshold, as previously described (9). (Inset) Representative PCR amplification curves. PCR cycles are represented on the x axis, whereas the y axis represents log mean fluorescence. (B) p63 specifically associates with C40 in intact skin. Chromatin extracted from cross-linked newborn mouse skin was immunoprecipitated in parallel using anti-p63, anti-p53, or anti-ERK (ctr) antibodies. The C40 enhancer and the distal genomic region were amplified. (C) p63 proteins affect expression of the endogenous p63 gene. HeLa cells were transiently transfected with expression vectors for the indicated proteins. The mRNA levels of the endogenous p63 transcripts were quantified by real-time RT-PCR using oligonucleotides directed to the 5′ untranslated region of the human ΔNp63 mRNA. Values are expressed as induction (n-fold) of the control transfection. Transfection efficiency was normalized by cotransfecting an expression vector for green fluorescent protein (GFP). (D) Exogenous expression of ΔNp63γ in HeLa cells turns on expression of the endogenous ΔNp63α protein. HeLa cells were transiently transfected as described for panel C. Immunoblotting of total cell extracts was performed using anti-p63 antibodies. As a control, an extract of HeLa cells transfected with ΔNp63α was also run at the same time. Due to the higher stability of ΔNp63α than of ΔNp63γ, approximately one-fifth as much protein extract was loaded. Membranes were reprobed with anti-ERK antibodies as a loading control.